Abstract
Many articles on COVID19 deaths have been published since the pandemic has occurred. On reviewing the articles published until June 2021, the findings were very heterogeneous. Adding to the existing knowledge, there were also some unique observations made in the pathogenesis of COVID19. This review was done to determine the findings obtained and inferences drawn from various studies published globally among patients who died due to COVID19. PRISMA guidelines were used to conduct this systematic review. A search of databases like PubMed, ScienceDirect and Epistemonikos was done. The articles focusing on postmortem sample studies involving full autopsies, minimally invasive autopsies and tissue biopsy studies were screened and searched. The studies included were all the case reports, case series, narrative reviews and systematic reviews obtained in full text and in the English language containing study information, and samples obtained postmortem. The information obtained was tabulated using Microsoft excel sheets. The duplicates were removed at the beginning of the tabulation. Zotero referencing software was used for article sorting and citation and bibliography. Two authors independently reviewed the articles throughout the process to prevent bias. Adding to the heterogeneity of COVID19, the concept of lethality in preexisting disease conditions, the occurrence of secondary bacterial and fungal infections, and other pathogenetic mechanisms uniquely encountered are to be considered in treating the patients. Also, the presence of SARS-CoV-2 postmortem is established and should be considered a hazard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The WHO Coronavirus (COVID19) live dashboard on 08 September 2021 displayed 221,134,742 confirmed cases including 4,574,089 deaths globally. The WHO weekly situation report displays that the global incidence of COVID19 cases remained stable over the month with over 4.4 million new cases reported between 30 August 2021 and 05 September 2021, with just 64,000 deaths. All regions reported a declining trend of a similar trend except in regions of the Americas where there was a 19% increase comparatively [1]. Many articles have been published since the pandemic has occurred. Though there was hesitancy in conduction of postmortem/autopsy examination on COVID19 deaths, many autopsy surgeons/pathologists have conducted autopsies to reveal the heterogeneity of COVID19. Upon reviewing the articles published until June 2021, the findings were more in the lungs, heart and coagulopathy in general. But adding to the existing knowledge there were also some unique observations made in the pathogenesis of COVID19.
Rationale and objective of the review
This review was done to determine the findings obtained and inferences drawn from various studies published globally among patients who died due to COVID19.
Methods
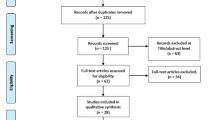
The current review was done according to Preferred Reporting Items for Systematic Review (PRISMA) guidelines [2]. A thorough search of databases including PubMed, ScienceDirect and Epistemonikos was done for the articles focusing on postmortem sample studies involving full autopsies, minimally invasive autopsies and tissue biopsy studies. The search terms used were “autopsy findings in SARS-CoV-2 deaths”, “forensic autopsy in COVID19 deaths”, “COVID19 histopathology findings” and “autopsy and COVID19 deaths”. The cross-references were done accordingly. The studies included were all the case reports, case series, narrative reviews, and systematic reviews obtained in full text and in the English language containing information on study samples obtained post-mortem. Few comparative studies were also selected. The articles with live sample study, without complete articles, and from other languages were excluded. Month /year wise studies with the author details, findings obtained, and the inferences drawn were tabulated using Microsoft Excel sheets. The duplicates were removed at the beginning of the tabulation. Zotero referencing software was used for article sorting and citation and bibliography. Two authors independently reviewed the articles throughout the process to prevent bias. (please see Fig. 1).
The findings of the study are summarized below (please see Table 1).
Discussion
COVID19 disease in general
The COVID19 displayed heterogeneity with organ involvement and with no specific viral injury [3]. Deaths in COVID19 are multifactorial in preexisting morbidity where COVID19 acts as a contributory factor for the fatal outcome [46, 97]. All the younger patients, and other individuals having preexisting health conditions are prone to thromboembolic complications, immune dysregulation, and liver damage and other contributing influences of COVID19 than direct contribution [4, 5, 107].
A study suggested that a panel of cytokines could be used to predict disease deterioration. Severe damage was attributed to both cytopathy and immunopathologic damage [6], and worsening of the disease did not require active SARS-CoV-2 infection [7].
A case series concluded that diffuse alveolar damage (DAD), thrombosis, haemophagocytosis and immune cell depletion are the interrelated pathological processes seen in COVID19. In addition, new autopsy findings were acute pancreatitis, adrenal micro-infarction, pericarditis, disseminated mucormycosis, aortic dissection, and marantic endocarditis. Active viral replication was noted outside the respiratory tract [8]. The thrombi formation was 9 times more prevalent with COVID19 than with influenza [9] and the thrombogenicity of SARS-CoV-2 infection is linked to widespread endothelial damage [10].
In later stages of the disease course, the virus was sporadically present indicating the maladaptive immune response causing further progress in the disease and that immunomodulation should be the target of therapy [11]. Usage of steroids in the critically ill was suggested [95].
The COVID19 induced coagulopathy indicated the therapeutic measures to target the coagulopathy [12]. Other systematic reviews also documented that major findings in lungs were diffuse alveolar damage, hyaline membrane formation, and microthrombi in small blood vessels [88, 96].
There was a high incidence of deep vein thrombosis and pulmonary embolism suggestive of endothelial involvement [96]. Other organs like the heart, liver, kidney, brain, spleen, skin, and adrenals displayed inflammation and vascular damage. Massive activation of the immune system and microvascular damage were found in COVID19 [88]. DAD of the lungs was superimposed with acute bronchopneumonia. Microthrombi were described in the placenta, lungs, kidneys, and central nervous system (CNS). The gastrointestinal tract displayed minimal such changes. The endothelial injury was commonly seen in the lungs. SARS-CoV-2 viral particles were demonstrated in organ-specific cells in the trachea, lungs, liver, large intestine, kidney, and CNS [13]. Frequent testing and strict surveillance of systemic parameters were recommended owing to the rapid spread of the disease [91, 97].
LUNGS
The lungs were the major target organ of severe COVID19 pneumonia with DAD and segmental pulmonary arterial thrombosis. DAD was typical exudative and proliferative phases of acute lung injury. The exudative DAD was seen within 8 days of disease and advanced stages after 17 days. The DAD occurring in COVID19 is morphologically not different from any other causes. The DAD had different phases – exudative, proliferative, and early repair phases. Hyaline membrane formation, type 2 pneumocyte hyperplasia, and acute fibrinous organizing pneumonia (AFOP) pattern were seen. There were multinucleated giant cells, smudge cells and vascular thrombosis present [14,15,16,17,18,19,20,21,22,23,24,25, 99, 101, 102].
SARS-CoV-2 viral particles primarily damaged the type 2 pneumocytes and caused thrombophilic activity [26,27,28]. Organotropism was highly seen in the lungs and the reticuloendothelial system [29].
The virus initiates direct damage in the acute phase and gets cleared by the body’s immune response in the organizing phase. The period of presence from infection is approximately 10 days, absent in the organizing phase of the disease [30, 31]. Viral RNA was found in the lungs and virus particles in endothelial cells and pneumocytes. The early onset pro-inflammatory, activation of the complement pathway/coagulation cascade resulting in systemic procoagulant state and endothelial expression of cytokines and alveolar macrophage infection by SARS-CoV-2 lead to cytokine storm and thrombotic microangiopathy, as well as damage to multiple organs [22, 32, 33].
The COVID19 is a unique disease characterized by extensive lung thrombosis and long-term viral RNA persistence in pneumocytes and endothelial cells – with the presence of infected cell syncytia [34]. In a few there was massive bilateral alveolar damage in the early acute respiratory distress syndrome (ARDS) which correlated with severe disease onset and progressive deterioration leading to death. Type 2 pneumocyte hyperplasia with atypia was seen. There were no inclusion bodies present [35]. SARS-CoV-2 was detected in airways and pneumocytes by immunohistochemical staining and electron microscopy for virions [36]. In a study the virus was detected in alveolar macrophages but not in extrapulmonary tissues [103]. Interestingly an observation was made on the presence of coronavirus-like particles in the kidneys and GIT other than the respiratory system by immunohistochemistry and ultrastructural examination using electron microscopy [37]. Co-infections like respiratory viral and bacterial infections were seen [103]. Coagulopathy in the lungs like pulmonary artery embolism and overall deep vein thrombosis was present [20, 36, 38].
The deaths that occurred in the second week were related to SARS-CoV-2 pneumonia. The deaths occurring earlier were noted from heart failure and those occurring later were due to complications [39].
Radiologically the ground glass appearances seen in postmortem CT dependent portions were nonspecific; whereas ill-defined round opacities, traction bronchiectasis and reverse halo sign can be considered key findings in COVID19 [40]. In some, lungs showed features of global multifocal reticular consolidation in the postmortem CT [41, 42].
The response to systemic thrombolysis was low because of inflammatory and prothrombotic changes in the arterial wall resulting in a lack of lung perfusion [43]. The deceased who were treated for a longer duration did not show capillaritis, vasculitis or endotheliitis, but thrombosis was majorly present showing advanced stages of organization. Unique finding obtained in a study was the presence of invasive mycosis and florid pneumonia in the areas of patchy DAD, and the presence of aspergillosis, and mucormycosis [44]. On the contrary, there was no evidence of invasive aspergillosis in a set of critically ill patients [45].
In one study, severe secondary bacterial infections were seen in the diabetic patients and also severe illness in preexisting disease conditions [89]. The treatment of COVID19 patients should also aim at the secondary acute bronchopneumonia and aspiration pneumonia developed during the course [21, 46].
Heart
In the heart the findings observed were inconsistent. Some displayed direct injury of the heart by the virus, some did not. The COVID19 cases frequently had cardiac fibrin microthrombi but there was no evidence of direct myocardial infection [47]. In one study less than 50% of SARS-CoV-2 were detected in the myocardium causing cardiac dilatation, ischemia, and mural microthrombi [48]. Individual case studies reported that the SARS-CoV-2 causes fulminant myocarditis[49, 50], on the contrary, one case report ruled out the virus causing the same [51].
The concept of direct injury of the heart and lungs and the procoagulant stage created by the virus was supported by a study [52].
A study solely focusing on the heart revealed that COVID19 leads to small vessel endotheliitis in the heart [53]. In another study [103], there was no extrapulmonary tissue-specific virus finding but the cardiac diseases were aggravated during COVID19 in a cohort [33], whereas in a study in children, cardiac dysfunction with COVID19 was due to myocardial stunning/oedema associated with the systemic inflammatory state and direct myocardial injury by SARS-CoV-2, and hypoxia was attributed as secondary to viral pneumonia. [54].
Brain
Data obtained indicated that SARS-CoV-2-related brain injury may be due to several pathogenetic mechanisms or due to direct viral effects [55]. A case demonstrated the CNS complications in COVID19 patients, providing potential parainfectious processes affecting the patients [98]. The neuropathological changes were mild and found no evidence of brain damage directly by SARS-CoV-2 [56]. There were various hypoxic-related neuropathological changes in the brain but no neurotropism was seen [90]. However, a study demonstrated that cerebrovascular accidents can be associated with COVID19 [57].
Kidneys
The data provided evidence of direct kidney injury – the presence of a cluster of coronavirus particles in the tubular epithelium with upregulation of ACE2 receptors causing acute kidney injury (AKI) [58, 100]. However, the AKI was mild, suggestive of potential reversible kidney damage [59].
Muscle
The skeletal muscle displayed damage by the viral cytopathic effect and by elevated cytokines [60].
Teeth
SARS-CoV-2 was demonstrated in the periodontal tissue [61].
Testes
The testes exhibited significant seminiferous tubular injury, reduced Leydig cells and mild lymphocytic inflammation; however, there was no evidence of SARS-CoV-2 virus in the testes [62] Supporting it there was impaired spermatogenesis in COVID19 and in addition occurrence of autoimmune orchitis [63], suggesting precautions in the process of sperm donation.
Liver
COVID19 is not associated with any liver-specific histopathology [64] Aiding to it, a study confirmed that liver failure was not the main target of COVID19. Whatever derangement of the intrahepatic blood vessel network displayed, it was secondary to systemic changes caused by the virus [65].
Eyes
As analysis of three different sequences – RdRo-gene, E-gene, and Orf1 gene – the existence of SARS-CoV-2 viral RNA was proved to be present in the retina. [66].
Fetus
A spontaneous miscarriage occurring in a primigravida who was positive for SARS-CoV-2 in the nasal, placental, umbilical cord, and amniotic fluid swabs during labour did not rule out local bacterial infection [67]. Similarly, there was a case of foetal demise in a woman confirmed with SARS-CoV-2 infection without any other causes in COVID19 pregnancies [68]. In one study confirmative foetal death as an outcome of SARS-CoV-2 infection in pregnancy was displayed with direct infection of SARS-CoV-2 on the placenta [92].
In HIV patients
In the HIV-positive patient, a study[69] did not show any specific findings with COVID19.
Some of the unique findings obtained which are applicable in general
-
Ferroptosis was the proposed cause of ischemia–reperfusion injury in COVID19 causing cardiac and multiple organ injury [70].
-
The endothelial dysfunction and pyroptosis pathway were the cause of systemic thrombotic events [71].
-
The organ damage was due to extensive NETs and vascular damage [11, 72].
-
HA (glycosaminoglycan hyaluronan) was demonstrated in the alveolar spaces in the COVID19 lung in lethal cases. Advised adjuvant therapy targeting HA may be used in treating COVID19 [73].
-
Viral RNA was detected even in formalin-fixed paraffin-embedded tissues of the lungs, airways, lymph nodes, and spleen [93].
-
Platelet-rich fibrin microthrombi play a significant role in systemic coagulopathy [74].
-
SARS CoV-2 is associated with haemophagocytic lymphohistiocytosis (HLH). Identification of HLH will be useful for therapeutic strategies [75].
-
Demonstrated galactomannan antigen – confirmed invasive pulmonary aspergillosis (IPA). Recommended further assessment of the frequency of occurrence of IPA [76].
-
Lesions of histiocytic hyperplasia with haemophagocytosis (HHH) in the majority of cases demonstrated that COVID19 triggers a systemic immune-inflammatory disease [77].
Conclusions
As displayed by various studies, the effect of SARS-CoV-2 is heterogeneous. It affected highly the lungs, followed by the heart in its direct effect. Other organs including the brain, kidney, skin, skeletal muscle, eyes, and testes were seen affected by the disease. The liver and gastrointestinal tract were minimally affected. The disease progression was rapid and more deteriorating in the patients having preexisting medical conditions. In this review, there were ferroptosis, pyroptosis, NETs and platelet-rich fibrin microthrombi identified as unique pathogenetic mechanisms. The presence of histiocytic hyperplasia with haemophagocytosis (HHH), hemophagocytic lymphohistiocytosis (HLH), and glycosaminoglycan hyaluronan (HA) in lethal cases was observed. The occurrence of secondary bacterial infections and invasive pulmonary aspergillosis should be considered in the therapeutic approach. Special attention should be given to the COVID19 pregnancies. It is observed that a full autopsy cannot be replaced by minimal autopsy techniques. The studies also supported that a forensic pathologist should always be suspicious of the pathogen in the pandemics while conducting any medicolegal autopsy.
The concerning factor is that the findings are so heterogenous in the organs involved and the pathology seen that the definitive course of the disease cannot be decided in an individual who has developed COVID19.
Key points
-
1.
The lungs followed by the heart are greatly affected directly by the virus.
-
2.
Disease progresses rapidly in patients having preexisting medical conditions.
-
3.
This review obtained information about ferroptosis, pyroptosis, neutrophil extracellular traps (NETs) and platelet-rich fibrin microthrombi as unique pathogenetic mechanisms in COVID19.
-
4.
The presence of histiocytic hyperplasia with hemophagocytosis (HHH), haemophagocytosis lymphohistiocytosis (HLH) and glycosaminoglycan hyaluronan (HA) in lethal cases was observed.
-
5.
Secondary bacterial infections and invasive pulmonary aspergillosis should be considered in the therapeutic approach.
References
Weekly epidemiological update on COVID-19 - 7 September 2021 [Internet]. [cited 2021 Sep 8]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---7-september-2021
PRISMA [Internet]. [cited 2021 Sep 8]. Available from: http://prisma-statement.org/PRISMAStatement/Checklist
Remmelink M, De Mendonça R, D'Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020 Aug 12;24(1):495. https://doi.org/10.1186/s13054-020-03218-5. PMID: 32787909; PMCID: PMC7422463.
Attoh S, Segborwotso RP, Akoriyea SK, Teddy G, Edusei L, Hobenu F, Agyemang-Bediako K, Toppar A, Fatchu RD, Akakpo PK. COVID-19 autopsy reports from the Ga-East Municipal and the 37 Military Hospitals in Accra, Ghana. Ghana Med J. 2020 Dec;54(4 Suppl):52-61. https://doi.org/10.4314/gmj.v54i4s.9. PMID: 33976442; PMCID: PMC8087362.
Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, Sinn BV, Gerhold M, Hauptmann K, Ingold-Heppner B, Miller F, Herbst H, Corman VM, Martin H, Radbruch H, Heppner FL, Horst D. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021 Feb 19;11(1):4263. https://doi.org/10.1038/s41598-021-82862-5. PMID: 33608563; PMCID: PMC7895917.
Li S, Jiang L, Li X, Lin F, Wang Y, Li B, Jiang T, An W, Liu S, Liu H, Xu P, Zhao L, Zhang L, Mu J, Wang H, Kang J, Li Y, Huang L, Zhu C, Zhao S, Lu J, Ji J, Zhao J. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020 Jun 18;5(12):e138070. https://doi.org/10.1172/jci.insight.138070. PMID: 32427582; PMCID: PMC7406259.
Shao C, Liu H, Meng L, Sun L, Wang Y, Yue Z, Kong H, Li H, Weng H, Lv F, Jin R. Evolution of severe acute respiratory syndrome coronavirus 2 RNA test results in a patient with fatal coronavirus disease 2019: a case report. Hum Pathol. 2020 Jul;101:82-88. https://doi.org/10.1016/j.humpath.2020.04.015. Epub 2020 May 11. PMID: 32437706; PMCID: PMC7211665.
Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 Oct;1(6):e245-e253. https://doi.org/10.1016/S2666-5247(20)30115-4. Epub 2020 Aug 20. PMID: 32844161; PMCID: PMC7440861.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120-128. https://doi.org/10.1056/NEJMoa2015432. Epub 2020 May 21. PMID: 32437596; PMCID: PMC7412750.
Cipolloni L, Sessa F, Bertozzi G, Baldari B, Cantatore S, Testi R, D'Errico S, Di Mizio G, Asmundo A, Castorina S, Salerno M, Pomara C. Preliminary Post-Mortem COVID-19 Evidence of Endothelial Injury and Factor VIII Hyperexpression. Diagnostics (Basel). 2020 Aug 9;10(8):575. https://doi.org/10.3390/diagnostics10080575. PMID: 32784826; PMCID: PMC7460315.
Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, Fronczek J, Heunks LMA, de Jong MD, Guo L, du Long R, Lutter R, Molenaar PCG, Neefjes-Borst EA, Niessen HWM, van Noesel CJM, Roelofs JJTH, Snijder EJ, Soer EC, Verheij J, Vlaar APJ, Vos W, van der Wel NN, van der Wal AC, van der Valk P, Bugiani M. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020 Nov;1(7):e290-e299. https://doi.org/10.1016/S2666-5247(20)30144-0. Epub 2020 Sep 25. PMID: 33015653; PMCID: PMC7518879.
Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020 Aug 18;173(4):268-277. https://doi.org/10.7326/M20-2003. Epub 2020 May 6. PMID: 32374815; PMCID: PMC7240772.
Peiris S, Mesa H, Aysola A, Manivel J, Toledo J, Borges-Sa M, Aldighieri S, Reveiz L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS One. 2021 Apr 28;16(4):e0250708. https://doi.org/10.1371/journal.pone.0250708. PMID: 33909679; PMCID: PMC8081217.
Mauad T, Duarte-Neto AN, da Silva LFF, de Oliveira EP, de Brito JM, do Nascimento ECT, de Almeida Monteiro RA, Ferreira JC, de Carvalho CRR, do Nascimento Saldiva PH, Dolhnikoff M. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir Res. 2021 Jan 29;22(1):32. https://doi.org/10.1186/s12931-021-01628-9. PMID: 33514373; PMCID: PMC7844838.
Arslan MN, Büyük Y, Ziyade N, Elgörmüş N, Şirin G, Çoban İ, Gökşen ME, Daş T, Akçay A. COVID-19 autopsies of Istanbul. Ir J Med Sci. 2022 Apr;191(2):529-541. https://doi.org/10.1007/s11845-021-02602-6. Epub 2021 Mar 23. PMID: 33755916; PMCID: PMC7985574.
Sadegh Beigee F, Pourabdollah Toutkaboni M, Khalili N, Nadji SA, Dorudinia A, Rezaei M, Askari E, Farzanegan B, Marjani M, Rafiezadeh A. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol Res Pract. 2020 Oct;216(10):153228. https://doi.org/10.1016/j.prp.2020.153228. Epub 2020 Sep 19. PMID: 32979740; PMCID: PMC7837112.
Yan L, Mir M, Sanchez P, Beg M, Peters J, Enriquez O, Gilbert A. COVID-19 in a Hispanic Woman. Arch Pathol Lab Med. 2020 Sep 1;144(9):1041-1047. https://doi.org/10.5858/arpa.2020-0217-SA. PMID: 32422081.
Aguiar D, Lobrinus JA, Schibler M, Fracasso T, Lardi C. Inside the lungs of COVID-19 disease. Int J Legal Med. 2020 Jul;134(4):1271-1274. https://doi.org/10.1007/s00414-020-02318-9. Epub 2020 May 26. PMID: 32458044; PMCID: PMC7248187.
Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, Nishioka H, Akita H, Sato Y, Kataoka M, Katano H, Tobiume M, Sekizuka T, Itokawa K, Kuroda M, Suzuki T. Clinicopathologic and Immunohistochemical Findings from Autopsy of Patient with COVID-19, Japan. Emerg Infect Dis. 2020 Sep;26(9):2157–61. https://doi.org/10.3201/eid2609.201353. Epub 2020 May 15. PMID: 32412897; PMCID: PMC7454070.
Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020 Jul;134(4):1275-1284. https://doi.org/10.1007/s00414-020-02317-w. Epub 2020 Jun 4. Erratum in: Int J Legal Med. 2020 Sep;134(5):1977. PMID: 32500199; PMCID: PMC7271136.
Wu JH, Li X, Huang B, Su H, Li Y, Luo DJ, Chen S, Ma L, Wang SH, Nie X, Peng L. [Pathological changes of fatal coronavirus disease 2019 (COVID-19) in the lungs: report of 10 cases by postmortem needle autopsy]. Zhonghua Bing Li Xue Za Zhi. 2020 Jun 8;49(6):568-575. Chinese. https://doi.org/10.3760/cma.j.cn112151-20200405-00291. PMID: 32486534.
Bösmüller H, Traxler S, Bitzer M, Häberle H, Raiser W, Nann D, Frauenfeld L, Vogelsberg A, Klingel K, Fend F. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020 Sep;477(3):349-357. https://doi.org/10.1007/s00428-020-02881-x. Epub 2020 Jun 30. PMID: 32607684; PMCID: PMC7324489.
Schwensen HF, Borreschmidt LK, Storgaard M, Redsted S, Christensen S, Madsen LB. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J Clin Pathol. 2020 Jul 28:jclinpath-2020-206879. https://doi.org/10.1136/jclinpath-2020-206879. Epub ahead of print. PMID: 32723800.
Grosse C, Grosse A, Salzer HJF, Dünser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020 Nov-Dec;49:107263. https://doi.org/10.1016/j.carpath.2020.107263. Epub 2020 Jul 16. PMID: 32784110; PMCID: PMC7365076.
Navarro Conde P, Alemany Monraval P, Medina Medina C, Jiménez Sánchez A, Andrés Teruel JC, Ferrando Marco J, Puglia Santos V, Mayordomo Aranda E. Autopsy findings from the first known death from Severe Acute Respiratory Syndrome SARS-CoV-2 in Spain. Rev Esp Patol. 2020 Jul-Sep;53(3):188-192. https://doi.org/10.1016/j.patol.2020.04.002. Epub 2020 May 11. PMID: 32650970; PMCID: PMC7211676.
Tombolini A, Scendoni R. SARS-CoV-2-related deaths in routine forensic autopsy practice: histopathological patterns. Int J Legal Med. 2020 Nov;134(6):2205-2208. https://doi.org/10.1007/s00414-020-02354-5. Epub 2020 Jun 29. PMID: 32613447; PMCID: PMC7327488.
Okudela K, Hayashi H, Yoshimura Y, Sasaki H, Horiuchi H, Miyata N, Tachikawa N, Tsuchiya Y, Mitsui H, Ohashi K. A Japanese case of COVID-19: An autopsy report. Pathol Int. 2020 Oct;70(10):820-824. https://doi.org/10.1111/pin.13002. Epub 2020 Aug 13. PMID: 32794245; PMCID: PMC7436745.
Oprinca GC, Muja LA. Postmortem examination of three SARS-CoV-2-positive autopsies including histopathologic and immunohistochemical analysis. Int J Legal Med. 2021 Jan;135(1):329-339. https://doi.org/10.1007/s00414-020-02406-w. Epub 2020 Aug 27. PMID: 32851474; PMCID: PMC7449785.
Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, Millar T, Lerpiniere CEB, Tagliavini G, Hartley CS, Randle NP, Gachanja NN, Potey PMD, Dong X, Anderson AM, Campbell VL, Duguid AJ, Al Qsous W, BouHaidar R, Baillie JK, Dhaliwal K, Wallace WA, Bellamy COC, Prost S, Smith C, Hiscox JA, Harrison DJ, Lucas CD. Tissue-Specific Immunopathology in Fatal COVID-19. Am J Respir Crit Care Med. 2021 Jan 15;203(2):192-201. https://doi.org/10.1164/rccm.202008-3265OC. PMID: 33217246; PMCID: PMC7874430.
Schaefer IM, Padera RF, Solomon IH, Kanjilal S, Hammer MM, Hornick JL, Sholl LM. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. 2020 Nov;33(11):2104-2114. https://doi.org/10.1038/s41379-020-0595-z. Epub 2020 Jun 19. PMID: 32561849; PMCID: PMC7304376.
Sauter JL, Baine MK, Butnor KJ, Buonocore DJ, Chang JC, Jungbluth AA, Szabolcs MJ, Morjaria S, Mount SL, Rekhtman N, Selbs E, Sheng ZM, Xiao Y, Kleiner DE, Pittaluga S, Taubenberger JK, Rapkiewicz AV, Travis WD. Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology. 2020 Dec;77(6):915-925. https://doi.org/10.1111/his.14201. Epub 2020 Oct 16. PMID: 32614086; PMCID: PMC7361244.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020 Jun;220:1-13. https://doi.org/10.1016/j.trsl.2020.04.007. Epub 2020 Apr 15. PMID: 32299776; PMCID: PMC7158248.
Wang XX, Shao C, Huang XJ, Sun L, Meng LJ, Liu H, Zhang SJ, Li HJ, Lv FD. Histopathological features of multiorgan percutaneous tissue core biopsy in patients with COVID-19. J Clin Pathol. 2021 Aug;74(8):522-527. https://doi.org/10.1136/jclinpath-2020-206623. Epub 2020 Aug 26. PMID: 32848014; PMCID: PMC8311110.
Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, Volpe MC, Colliva A, Zanconati F, Berlot G, Silvestri F, Zacchigna S, Giacca M. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020 Nov;61:103104. https://doi.org/10.1016/j.ebiom.2020.103104. Epub 2020 Nov 3. PMID: 33158808; PMCID: PMC7677597.
Suess C, Hausmann R. Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Int J Legal Med. 2020 Jul;134(4):1285-1290. https://doi.org/10.1007/s00414-020-02319-8. Epub 2020 Jun 5. PMID: 32504146; PMCID: PMC7273129.
Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020 Oct;20(10):1135-1140. https://doi.org/10.1016/S1473-3099(20)30434-5. Epub 2020 Jun 8. PMID: 32526193; PMCID: PMC7279758.
Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020 Aug 1;396(10247):320-332. https://doi.org/10.1016/S0140-6736(20)31305-2. Epub 2020 Jul 16. Erratum in: Lancet. 2020 Aug 1;396(10247):312. PMID: 32682491; PMCID: PMC7365650.
Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020 Jul;8(7):681-686. https://doi.org/10.1016/S2213-2600(20)30243-5. Epub 2020 May 27. PMID: 32473124; PMCID: PMC7255143.
Malézieux-Picard A, Ferrer Soler C, De Macedo Ferreira D, Gaud-Luethi E, Serratrice C, Mendes A, Zekry D, Gold G, Lobrinus JA, Arnoux G, Serra F, Prendki V. Undetected Causes of Death in Hospitalized Elderly with COVID-19: Lessons from Autopsy. J Clin Med. 2021 Mar 24;10(7):1337. https://doi.org/10.3390/jcm10071337. PMID: 33804890; PMCID: PMC8037274.
Helmrich E, Decker L, Adolphi N, Makino Y. Postmortem CT lung findings in decedents with Covid-19: A review of 14 decedents and potential triage implications. Forensic Imaging. 2020 Dec;23:200419. https://doi.org/10.1016/j.fri.2020.200419. Epub 2020 Nov 5. PMCID: PMC7643627.
Fitzek A, Sperhake J, Edler C, Schröder AS, Heinemann A, Heinrich F, Ron A, Mushumba H, Lütgehetmann M, Püschel K. Evidence for systematic autopsies in COVID-19 positive deceased: Case report of the first German investigated COVID-19 death. Rechtsmedizin (Berl). 2020;30(3):184-189. https://doi.org/10.1007/s00194-020-00401-4. Epub 2020 May 25. PMID: 32836897; PMCID: PMC7247437.
Heinrich F, Sperhake JP, Heinemann A, Mushumba H, Lennartz M, Nörz D, Glatzel M, Lütgehetmann M, Püschel K. Germany's first COVID-19 deceased: a 59-year-old man presenting with diffuse alveolar damage due to SARS-CoV-2 infection. Virchows Arch. 2020 Sep;477(3):335-339. https://doi.org/10.1007/s00428-020-02872-y. Epub 2020 Jul 4. PMID: 32666230; PMCID: PMC7359760.
Porembskaya O, Lobastov K, Pashovkina O, Tsaplin S, Schastlivtsev I, Zhuravlev S, Laberko L, Rodoman G, Kravchuk V, Skvortsov A, Saiganov S. Thrombosis of pulmonary vasculature despite anticoagulation and thrombolysis: The findings from seven autopsies. Thrombosis Update. 2020 Dec;1:100017. https://doi.org/10.1016/j.tru.2020.100017. Epub 2020 Oct 17. PMCID: PMC7568050.
Evert K, Dienemann T, Brochhausen C, Lunz D, Lubnow M, Ritzka M, Keil F, Trummer M, Scheiter A, Salzberger B, Reischl U, Boor P, Gessner A, Jantsch J, Calvisi DF, Evert M, Schmidt B, Simon M. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch. 2021 Jul;479(1):97-108. https://doi.org/10.1007/s00428-020-03014-0. Epub 2021 Jan 20. PMID: 33471172; PMCID: PMC7816067.
Flikweert AW, Grootenboers MJJH, Yick DCY, du Mée AWF, van der Meer NJM, Rettig TCD, Kant MKM. Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020 Oct;59:149-155. https://doi.org/10.1016/j.jcrc.2020.07.002. Epub 2020 Jul 8. PMID: 32674001; PMCID: PMC7340597.
Anja C. Roden, MD, Melanie C. Bois, MD, Tucker F. Johnson, MD, Marie Christine Aubry, MD, Mariam P. Alexander, MD, Catherine E. Hagen, MD, Peter T. Lin, MD, Reade A. Quinton, MD, Joseph J. Maleszewski, MD, Jennifer M. Boland, MD. The Spectrum of Histopathologic Findings in Lungs of Patients With Fatal Coronavirus Disease 2019 (COVID-19) Infection. Arch Pathol Lab Med (2021) 145 (1): 11–21. https://doi.org/10.5858/arpa.2020-0491-SA
Melanie C. Bois, Nicholas A. Boire, Andrew J. Layman, Marie-Christine Aubry, Mariam P. Alexander, Anja C. Roden, Catherine E. Hagen, Reade A. Quinton, Christopher Larsen, Young Erben, Ramanath Majumdar, Sarah M. Jenkins, Benjamin R. Kipp, Peter T. Lin, Joseph J. Maleszewski. COVID-19–Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation. 2021;143:230–243. https://doi.org/10.1161/CIRCULATIONAHA.120.050754
Roshdy A, Zaher S, Fayed H, Coghlan JG. COVID-19 and the Heart: A Systematic Review of Cardiac Autopsies. Front Cardiovasc Med. 2021 Jan 28;7:626975. https://doi.org/10.3389/fcvm.2020.626975. PMID: 33585586; PMCID: PMC7876291.
Gauchotte G, Venard V, Segondy M, Cadoz C, Esposito-Fava A, Barraud D, Louis G. SARS-Cov-2 fulminant myocarditis: an autopsy and histopathological case study. Int J Legal Med. 2021 Mar;135(2):577-581. https://doi.org/10.1007/s00414-020-02500-z. Epub 2021 Jan 3. PMID: 33392658; PMCID: PMC7779100.
Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. 2020 Jun;39(3):263-268. https://doi.org/10.1080/15513815.2020.1761491. Epub 2020 May 13. PMID: 32401577; PMCID: PMC7232882.
Beigmohammadi MT, Jahanbin B, Safaei M, Amoozadeh L, Khoshavi M, Mehrtash V, Jafarzadeh B, Abdollahi A. Pathological Findings of Postmortem Biopsies From Lung, Heart, and Liver of 7 Deceased COVID-19 Patients. Int J Surg Pathol. 2021 Apr;29(2):135-145. https://doi.org/10.1177/1066896920935195. Epub 2020 Jun 19. PMID: 32552178; PMCID: PMC8041443.
Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020 Sep-Oct;48:107233. https://doi.org/10.1016/j.carpath.2020.107233. Epub 2020 May 7. PMID: 32434133; PMCID: PMC7204762.
Maccio U, Zinkernagel AS, Shambat SM, Zeng X, Cathomas G, Ruschitzka F, Schuepbach RA, Moch H, Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021 Jan;63:103182. https://doi.org/10.1016/j.ebiom.2020.103182. Epub 2021 Jan 7. PMID: 33422990; PMCID: PMC7808909.
Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, Figueiredo Delgado A, Montanari Fiorita C, Nunes Leal G, Rodrigues RM, Taverna Chaim K, Rebello Pinho JR, Carneiro-Sampaio M, Mauad T, Ferraz da Silva LF, Brunow de Carvalho W, Saldiva PHN, Garcia Caldini E. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020 Oct;4(10):790-794. https://doi.org/10.1016/S2352-4642(20)30257-1. Epub 2020 Aug 20. Erratum in: Lancet Child Adolesc Health. 2020 Oct;4(10):e39. PMID: 32828177; PMCID: PMC7440866.
Fabbri VP, Foschini MP, Lazzarotto T, Gabrielli L, Cenacchi G, Gallo C, Aspide R, Frascaroli G, Cortelli P, Riefolo M, Giannini C, D'Errico A. Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. 2021 Jan;31(1):205-210. https://doi.org/10.1111/bpa.12901. Epub 2020 Nov 2. PMID: 33002281; PMCID: PMC7536900.
Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M, Heinemann A, Pfefferle S, Schwabenland M, Sumner Magruder D, Bonn S, Prinz M, Gerloff C, Püschel K, Krasemann S, Aepfelbacher M, Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020 Nov;19(11):919-929. https://doi.org/10.1016/S1474-4422(20)30308-2. Epub 2020 Oct 5. PMID: 33031735; PMCID: PMC7535629.
Taylor LD, Ameen OS, Zaharie SD. Complete Clinicopathological Case Report of a Young Patient Dying of COVID-19-Related Stroke. Am J Forensic Med Pathol. 2021 Jun 1;42(2):160-163. https://doi.org/10.1097/PAF.0000000000000668. PMID: 33491953; PMCID: PMC8115422.
Farkash EA, Wilson AM, Jentzen JM. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J Am Soc Nephrol. 2020 Aug;31(8):1683-1687. https://doi.org/10.1681/ASN.2020040432. Epub 2020 May 5. Erratum in: J Am Soc Nephrol. 2020 Oct;31(10):2494. PMID: 32371536; PMCID: PMC7460898.
Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D'Agati V, Markowitz G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J Am Soc Nephrol. 2020 Sep;31(9):2158-2167. https://doi.org/10.1681/ASN.2020050744. Epub 2020 Jul 29. PMID: 32727719; PMCID: PMC7461662.
Mageriu V, Zurac S, Bastian A, Staniceanu F, Manole E. Histological findings in skeletal muscle of SARS-CoV2 infected patient. J Immunoassay Immunochem. 2020 Nov 1;41(6):1000-1009. https://doi.org/10.1080/15321819.2020.1863819. Epub 2020 Dec 22. PMID: 33353460.
Adam M. Is SARS-CoV-2 present in the periodontium? A post-mortem study. Evid Based Dent. 2021 Jan;22(2):60-61. https://doi.org/10.1038/s41432-021-0184-0. PMID: 34172907; PMCID: PMC8226333.
Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF, Li X, Zhou JJ, Fan J, Luo DJ, Chang XN, Arkun K, Zhou M, Nie X. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur Urol Focus. 2020 Sep 15;6(5):1124-1129. https://doi.org/10.1016/j.euf.2020.05.009. Epub 2020 May 31. PMID: 32563676; PMCID: PMC7261470.
Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K, Zhou L, Shen S, Liu L, Liu Q, Xiong C. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020 Nov;28:100604. https://doi.org/10.1016/j.eclinm.2020.100604. Epub 2020 Oct 23. PMID: 33134901; PMCID: PMC7584442.
Schmit G, Lelotte J, Vanhaebost J, Horsmans Y, Van Bockstal M, Baldin P. The Liver in COVID-19-Related Death: Protagonist or Innocent Bystander? Pathobiology. 2021;88(1):88-94. https://doi.org/10.1159/000512008. Epub 2020 Oct 27. PMID: 33108789; PMCID: PMC7705929.
Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020 Sep;40(9):2110-2116. https://doi.org/10.1111/liv.14601. Epub 2020 Jul 20. PMID: 32654359; PMCID: PMC7404964.
Casagrande M, Fitzek A, Püschel K, Aleshcheva G, Schultheiss HP, Berneking L, Spitzer MS, Schultheiss M. Detection of SARS-CoV-2 in Human Retinal Biopsies of Deceased COVID-19 Patients. Ocul Immunol Inflamm. 2020 Jul 3;28(5):721-725. https://doi.org/10.1080/09273948.2020.1770301. Epub 2020 May 29. PMID: 32469258.
Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA. 2020 Jun 2;323(21):2198-2200. https://doi.org/10.1001/jama.2020.7233. PMID: 32352491; PMCID: PMC7193526.
Poisson TM, Pierone G Jr. Placental pathology and fetal demise at 35 weeks of gestation in a woman with SARS-CoV-2 infection: A case report. Case Rep Womens Health. 2021 Apr;30:e00289. https://doi.org/10.1016/j.crwh.2021.e00289. Epub 2021 Jan 28. PMID: 33527073; PMCID: PMC7840395.
Khaba MC, Ngale TC, Madala N. COVID-19 in an HIV-infected patient. Lessons learned from an autopsy case. Int J Infect Dis. 2020 Dec;101:243-246. https://doi.org/10.1016/j.ijid.2020.09.1435. Epub 2020 Sep 25. PMID: 32987179; PMCID: PMC7518850.
Jacobs W, Lammens M, Kerckhofs A, Voets E, Van San E, Van Coillie S, Peleman C, Mergeay M, Sirimsi S, Matheeussen V, Jansens H, Baar I, Vanden Berghe T, Jorens PG. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020 Sep 22;7(6):3772–81. https://doi.org/10.1002/ehf2.12958. Epub ahead of print. PMID: 32959998; PMCID: PMC7607145.
Nagashima S, Mendes MC, Camargo Martins AP, Borges NH, Godoy TM, Miggiolaro AFRDS, da Silva Dezidério F, Machado-Souza C, de Noronha L. Endothelial Dysfunction and Thrombosis in Patients With COVID-19-Brief Report. Arterioscler Thromb Vasc Biol. 2020 Oct;40(10):2404-2407. https://doi.org/10.1161/ATVBAHA.120.314860. Epub 2020 Aug 7. PMID: 32762443; PMCID: PMC7505138.
Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C, Kremer AN, Völkl S, Amann K, Evert K, Falkeis C, Wehrfritz A, Rieker RJ, Hartmann A, Kremer AE, Neurath MF, Muñoz LE, Schett G, Herrmann M. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020 Aug;58:102925. https://doi.org/10.1016/j.ebiom.2020.102925. Epub 2020 Jul 31. PMID: 32745993; PMCID: PMC7397705.
Hellman U, Karlsson MG, Engström-Laurent A, Cajander S, Dorofte L, Ahlm C, Laurent C, Blomberg A. Presence of hyaluronan in lung alveoli in severe Covid-19: An opening for new treatment options? J Biol Chem. 2020 Nov 6;295(45):15418-15422. https://doi.org/10.1074/jbc.AC120.015967. Epub 2020 Sep 25. PMID: 32978255; PMCID: PMC7650240.
Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020 Jun 25;24:100434. https://doi.org/10.1016/j.eclinm.2020.100434. PMID: 32766543; PMCID: PMC7316051.
Prilutskiy A, Kritselis M, Shevtsov A, Yambayev I, Vadlamudi C, Zhao Q, Kataria Y, Sarosiek SR, Lerner A, Sloan JM, Quillen K, Burks EJ. SARS-CoV-2 Infection-Associated Hemophagocytic Lymphohistiocytosis. Am J Clin Pathol. 2020 Sep 8;154(4):466-474. https://doi.org/10.1093/ajcp/aqaa124. PMID: 32681166; PMCID: PMC7454285.
Santana MF, Pivoto G, Alexandre MAA, Baía-da-Silva DC, Borba MGDS, Val FA, Brito-Sousa JD, Melo GC, Monteiro WM, Souza JVB, Pinheiro SB, Ferreira LCL, Naveca FG, Nascimento VA, Corado ALG, Hajjar LA, Silva Neto JR, Siva GAV, Pasqualotto AC, Lacerda MVG. Confirmed Invasive Pulmonary Aspergillosis and COVID-19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020;53:e20200401. https://doi.org/10.1590/0037-8682-0401-2020. Epub 2020 Jul 3. PMID: 32638890; PMCID: PMC7341831.
Prieto-Pérez L, Fortes J, Soto C, Vidal-González Á, Alonso-Riaño M, Lafarga M, Cortti MJ, Lazaro-Garcia A, Pérez-Tanoira R, Trascasa Á, Antonio A, Córdoba R, Rodríguez-Pinilla SM, Cedeño O, Peces-Barba G, Fernández-Ormaechea I, Díez Medrano MJ, López de Las Heras M, Cabello A, Petkova E, Álvarez B, Carrillo I, Silva AM, Castellanos M, Calpena S, Valverde-Monge M, Fresneda D, Rubio-Martín R, Cornejo I, Astilleros Blanco de Cordova L, de la Fuente S, Recuero S, Górgolas M, Piris MA. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020 Nov;33(11):2139-2146. https://doi.org/10.1038/s41379-020-0613-1. Epub 2020 Jul 3. PMID: 32620916; PMCID: PMC7333227.
Kommoss FKF, Schwab C, Tavernar L, Schreck J, Wagner WL, Merle U, Jonigk D, Schirmacher P, Longerich T. The Pathology of Severe COVID-19-Related Lung Damage. Dtsch Arztebl Int. 2020 Jul 20;117(29-30):500-506. https://doi.org/10.3238/arztebl.2020.0500. PMID: 32865490; PMCID: PMC7588618.
Bruce-Brand C, Allwood BW, Koegelenberg CFN, Lalla U, Louw E, Diacon AH, Schubert PT. Postmortem lung biopsies from four patients with COVID-19 at a tertiary hospital in Cape Town, South Africa. S Afr Med J. 2020 Oct 19;110(12):1195-1200. https://doi.org/10.7196/SAMJ.2020.v110i12.15290. PMID: 33403965.
Seetulsingh P, Kannangara CI, Richman P. Undetectable SARS-CoV-2 in a nasopharyngeal swab but persistent viral RNA from deep lung swabs: findings from an autopsy. BMJ Case Rep. 2020 Oct 31;13(10):e237446. https://doi.org/10.1136/bcr-2020-237446. PMID: 33130584; PMCID: PMC7783368.
Damiani S, Fiorentino M, De Palma A, Foschini MP, Lazzarotto T, Gabrielli L, Viale PL, Attard L, Riefolo M, D'Errico A. Pathological post-mortem findings in lungs infected with SARS-CoV-2. J Pathol. 2021 Jan;253(1):31-40. https://doi.org/10.1002/path.5549. Epub 2020 Nov 4. PMID: 32930394.
Stonoga ETS, de Almeida Lanzoni L, Rebutini PZ, Permegiani de Oliveira AL, Chiste JA, Fugaça CA, Prá DMM, Percicote AP, Rossoni A, Nogueira MB, de Noronha L, Raboni SM. Intrauterine Transmission of SARS-CoV-2. Emerg Infect Dis. 2021 Feb;27(2):638-641. https://doi.org/10.3201/eid2702.203824. Epub 2020 Nov 13. PMID: 33185524; PMCID: PMC7853547.
Rakislova N, Marimon L, Ismail MR, Carrilho C, Fernandes F, Ferrando M, Castillo P, Rodrigo-Calvo MT, Guerrero J, Ortiz E, Muñoz-Beatove A, Martinez MJ, Hurtado JC, Navarro M, Bassat Q, Maixenchs M, Delgado V, Wallong E, Aceituno A, Kim J, Paganelli C, Goco NJ, Aldecoa I, Martinez-Pozo A, Martinez D, Ramírez-Ruz J, Cathomas G, Haab M, Menéndez C, Ordi J. Minimally Invasive Autopsy Practice in COVID-19 Cases: Biosafety and Findings. Pathogens. 2021 Apr 1;10(4):412. https://doi.org/10.3390/pathogens10040412. PMID: 33915771; PMCID: PMC8065952.
Ren L, Liu Q, Wang R, Chen R, Ao Q, Wang X, Zhang J, Deng F, Feng Y, Wang G, Zhou Y, Li L, Liu L. Clinicopathologic Features of COVID-19: A Case Report and Value of Forensic Autopsy in Studying SARS-CoV-2 Infection. Am J Forensic Med Pathol. 2021 Jun 1;42(2):164-169. https://doi.org/10.1097/PAF.0000000000000644. PMID: 33464756; PMCID: PMC8115425.
Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro-Filho J, Pinho JRR, Gomes-Gouvêa MS, Salles APM, de Oliveira IRS, Mauad T, Saldiva PHN, Dolhnikoff M. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020 Aug;77(2):186-197. https://doi.org/10.1111/his.14160. Epub 2020 Jul 24. PMID: 32443177; PMCID: PMC7280721.
Griffin KJ. Autopsy in the time of COVID. Diagn Histopathol (Oxf). 2021 Mar;27(3):134-137. https://doi.org/10.1016/j.mpdhp.2020.12.002. Epub 2021 Jan 7. PMID: 33519971; PMCID: PMC7832425.
Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 Aug;77(2):198-209. https://doi.org/10.1111/his.14134. Epub 2020 Jul 5. PMID: 32364264; PMCID: PMC7496150.
Mondello C, Roccuzzo S, Malfa O, Sapienza D, Gualniera P, Ventura Spagnolo E, Di Nunno N, Salerno M, Pomara C, Asmundo A. Pathological Findings in COVID-19 as a Tool to Define SARS-CoV-2 Pathogenesis. A Systematic Review. Front Pharmacol. 2021 Apr 1;12:614586. https://doi.org/10.3389/fphar.2021.614586. PMID: 33867981; PMCID: PMC8047201.
Jackson NR, Zeigler K, Torrez M, Makino Y, Adolphi NL, Lathrop S, Decker L, Dvorscak L, Proe L, Paul ID, Zumwalt R, Jarrell H. New Mexico's COVID-19 Experience. Am J Forensic Med Pathol. 2021 Mar 1;42(1):1-8. https://doi.org/10.1097/PAF.0000000000000664. PMID: 33416234; PMCID: PMC7870043.
Kantonen J, Mahzabin S, Mäyränpää MI, Tynninen O, Paetau A, Andersson N, Sajantila A, Vapalahti O, Carpén O, Kekäläinen E, Kantele A, Myllykangas L. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020 Nov;30(6):1012-1016. https://doi.org/10.1111/bpa.12889. Epub 2020 Aug 28. PMID: 32762083; PMCID: PMC7436498.
Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020 Dec;73(12):840-844. https://doi.org/10.1136/jclinpath-2020-206710. Epub 2020 Jun 30. PMID: 32605920.
Richtmann R, Torloni MR, Oyamada Otani AR, Levi JE, Crema Tobara M, de Almeida Silva C, Dias L, Miglioli-Galvão L, Martins Silva P, Macoto Kondo M. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep Womens Health. 2020 Jul 12;27:e00243. https://doi.org/10.1016/j.crwh.2020.e00243. PMID: 32704477; PMCID: PMC7354271.
Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. Molecular Detection of SARS-CoV-2 Infection in FFPE Samples and Histopathologic Findings in Fatal SARS-CoV-2 Cases. Am J Clin Pathol. 2020 Jul 7;154(2):190-200. https://doi.org/10.1093/ajcp/aqaa091. PMID: 32451533; PMCID: PMC7314275.
Himwaze CM, Telendiy V, Maate F, Mupeta S, Chitalu C, Chanda D, Julius P, Mumba C, Marimo C, Hamukale A, Mulenga L, Shibemba AL, Zumla A, Mucheleng'anga LA. Post-mortem examination of Hospital Inpatient COVID-19 Deaths in Lusaka, Zambia - A Descriptive Whole-body Autopsy Series. Int J Infect Dis. 2021 Jul;108:363-369. https://doi.org/10.1016/j.ijid.2021.06.013. Epub 2021 Jun 17. PMID: 34146690; PMCID: PMC8215884.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422. https://doi.org/10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. Erratum in: Lancet Respir Med. 2020 Feb 25;: PMID: 32085846; PMCID: PMC7164771.
Maiese A, Manetti AC, La Russa R, Di Paolo M, Turillazzi E, Frati P, Fineschi V. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2021 Jun;17(2):279-296. https://doi.org/10.1007/s12024-020-00310-8. Epub 2020 Oct 7. PMID: 33026628; PMCID: PMC7538370.
Bulfamante GP, Perrucci GL, Falleni M, Sommariva E, Tosi D, Martinelli C, Songia P, Poggio P, Carugo S, Pompilio G. Evidence of SARS-CoV-2 Transcriptional Activity in Cardiomyocytes of COVID-19 Patients without Clinical Signs of Cardiac Involvement. Biomedicines. 2020 Dec 18;8(12):626. https://doi.org/10.3390/biomedicines8120626. PMID: 33352880; PMCID: PMC7767122.
Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 Jul;140(1):1-6. https://doi.org/10.1007/s00401-020-02166-2. Epub 2020 May 24. PMID: 32449057; PMCID: PMC7245994.
Barisione E, Grillo F, Ball L, Bianchi R, Grosso M, Morbini P, Pelosi P, Patroniti NA, De Lucia A, Orengo G, Gratarola A, Verda M, Cittadini G, Mastracci L, Fiocca R. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2021 Mar;478(3):471-485. https://doi.org/10.1007/s00428-020-02934-1. Epub 2020 Sep 28. PMID: 32989525; PMCID: PMC7521863.
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 Jul;98(1):219-227. https://doi.org/10.1016/j.kint.2020.04.003. Epub 2020 Apr 9. PMID: 32327202; PMCID: PMC7194105.
Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020 Sep 1;173(5):350-361. https://doi.org/10.7326/M20-2566. Epub 2020 May 14. PMID: 32422076; PMCID: PMC7249507.
Konopka KE, Nguyen T, Jentzen JM, Rayes O, Schmidt CJ, Wilson AM, Farver CF, Myers JL. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD. Histopathology. 2020 Oct;77(4):570-578. https://doi.org/10.1111/his.14180. Epub 2020 Sep 12. PMID: 32542743; PMCID: PMC7323403.
Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, Goldsmith CS, Silva-Flannery L, Seixas JN, Reagan-Steiner S, Uyeki T, Denison A, Bhatnagar J, Shieh WJ, Zaki SR; COVID-19 Pathology Working Group. Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States. Emerg Infect Dis. 2020 Sep;26(9):2005-2015. https://doi.org/10.3201/eid2609.202095. Epub 2020 May 21. PMID: 32437316; PMCID: PMC7454055.
Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, Nie X, Zhou L, Liu Z, Ren Y, Yuan L, Zhang Y, Zhang J, Liang L, Chen X, Liu X, Wang P, Han X, Weng X, Chen Y, Yu T, Zhang X, Cai J, Chen R, Shi ZL, Bian XW. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020 Jul;57:102833. https://doi.org/10.1016/j.ebiom.2020.102833. Epub 2020 Jun 20. PMID: 32574956; PMCID: PMC7305897.
Skok K, Stelzl E, Trauner M, Kessler HH, Lax SF. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Arch. 2021 Feb;478(2):343-353. https://doi.org/10.1007/s00428-020-02903-8. Epub 2020 Aug 20. PMID: 32815036; PMCID: PMC7438212.
Schweitzer W, Ruder T, Baumeister R, Bolliger S, Thali M, Meixner E, Ampanozi G. Implications for forensic death investigations from first Swiss post-mortem CT in a case of non-hospital treatment with COVID-19. Forensic Imaging. 2020 Jun;21:200378. https://doi.org/10.1016/j.fri.2020.200378. Epub 2020 Apr 18. PMCID: PMC7166113.
Greuel S, Ihlow J, Dragomir MP, Streit S, Corman VM, Haberbosch L, Winkler D, Meinhardt J, Aschman T, Schneider J, Trotsyuk I, Kunze CA, Maurer L, Radbruch H, Heppner FL, Horst D, Elezkurtaj S. COVID-19: Autopsy findings in six patients between 26 and 46 years of age. Int J Infect Dis. 2021 Jul;108:274-281. https://doi.org/10.1016/j.ijid.2021.05.069. Epub 2021 Jun 3. PMID: 34089883; PMCID: PMC8172269.
Author information
Authors and Affiliations
Contributions
Dr. Raviraj KG contributed to the conception and design, acquisition of data and analysis, and interpretation of data. Dr. Shobhana SS contributed to the planning and tabulation needed for the review.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raviraj, K.G., Shobhana, S.S. Findings and inferences from full autopsies, minimally invasive autopsies and biopsy studies in patients who died as a result of COVID19 — A systematic review. Forensic Sci Med Pathol 18, 369–381 (2022). https://doi.org/10.1007/s12024-022-00494-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-022-00494-1