Abstract
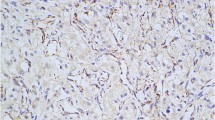
This is a confirmatory study about usefulness of SDHB and SDHA immunostaining in assessment of SDH mutations in paragangliomas and pheochromocytomas. Paraganglioma/pheochromocytoma syndrome (PGL/PCC syndrome) consists of different entities, associated with germline mutations in five different genes: SDHD, SDHAF2, SDHC, SDHA and SDHB. It has been suggested that negative immunostaining of SDHB can be taken as an indicator of the presence of a mutation in one of the five SDH genes. We have performed SDHB and SDHA immunohistochemical staining in a series of paragangliomas and pheochromocytomas from 64 patients. The patients had been previously checked for mutations in SDHD, SDHC and SDHB, but also for mutation in RET and VHL. All 14 patients with SDH mutations (9 with SDHB and 5 with SDHD mutations) exhibited negative or weak–diffuse SDHB staining pattern in tumour tissue, whereas cells of the 23 RET mutated and 8 VHL mutated tumours showed a positive SDHB immunostaining. Sixteen of the patients that did not exhibit a mutation in any gene showed positive SDHB immunostaining in tumour tissue, while only three of the patients without mutation exhibited negative staining. All patients exhibited positive pattern of SDHA immunostaining. The results confirm the value of SDHB immunohistochemical status in assessment of germline mutations in PGL/PCC syndrome.




Similar content being viewed by others
References
Neumann HPH, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292(8):943–51,2004.
Chen H, Sippel RS, O'Dorisio MS, et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 39(6):775–83, 2010.
Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and genotype phenotype proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat 31(1):41–51, 2010.
Eng C, Kiuru M, Fernandez MJ, et al. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer 3(3):193–202, 2003.
Komminoth P, Perren A, van Nederveen FH, et al. Familial endocrine tumours: phaeochromocytomas and extra-adrenal paragangliomas. Diagnostic Histopathology 15(2):61–8, 2009.
Nathanson K, Baysal BE, Drovdlic C et al. Familial paraganglioma-phaeocromocytoma syndromes caused by SDHB, SDHC and SDHB mutations. In: DeLellis R, Lloyd R, Heitz P, Eng C, eds. Pathology and Genetics of Tumours of Endocrine Organs (IARC WHO Classification of Tumours). World Health Organization 2004.
Benn DE. Clinical Presentation and Penetrance of Pheochromocytoma/Paraganglioma Syndromes. J Clin Endocrinol Metab 91(3):827–36, 2005.
van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol 10(8):764–71, 2009.
Kopetschke R, Slisko M, Kilisli A, et al. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 161(2):355–61, 2009.
O'Riordain DS, Young WF Jr, Grant CS, et al. Clinical Spectrum and Outcome of Functional Extraadrenal Paraganglioma. World J Surg 20(7):916–22, 1996.
Gill AJ, Benn DE, Chou A, et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol 41(6):805–14, 2010.
Korpershoek E, Favier J, Gaal J, et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab 96(9):1472–6, 2011.
Gimenez-Roqueplo AP. Functional Consequences of a SDHB Gene Mutation in an Apparently Sporadic Pheochromocytoma. J Clin Endocrinol Metab 87(10):4771–4, 2002.
Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 16(1):79–88, 2008.
Gimm O, Armanios M, Dziema H, et al. Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res 60(24):6822–5, 2000.
Burnichon N, Briere J-J, Libé R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 19(15):3011–20, 2010.
Qin Y, Yao L, King EE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet 42(3):229–33, 2010.
Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 43(7):663–7, 2011.
Neumann HP, Erlic Z, Boedeker C, et al. Clinical predictors for germline mutations in head and neck paraganglioma patients: Cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res 69(8):3650–6, 2009.
Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol 23(34):8812–8, 2005.
Schlisio S, Kenchappa RS, Vredeveld LC, et al. The kinesin KIF1B acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev 22(7):884–93, 2008.
Ladroue CC, Carcenac RR, Leporrier MM, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med 359(25):2685–92, 2008.
Welander J, Soderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer 18(6):253–76, 2011.
Cascon A, Pita G, Burnichon N, et al. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab 94(5):1701–5, 2009.
Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab 94(8):2817–27, 2009.
van Nederveen FH, Korpershoek E, Lenders JWM, et al. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N Engl J Med 357(3):306–8, 2007.
Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287(5454):848–51, 2000.
Blank A, Schmitt AM, Korpershoek E, et al. SDHB loss predicts malignancy in pheochromocytomas/sympathethic paragangliomas, but not through hypoxia signalling. Endocr Relat Cancer 17(4):919–28, 2010.
Bayley J-P, Kunst HPM, Cascon A, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol 11(4):366–72, 2010.
Schiavi F, Boedeker CC, Bausch B, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA 294(16):2057–63, 2005.
Guzy RD, Sharma B, Bell E, et al. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 28(2):718–31, 2008.
Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab 92(10):3822–8, 2007.
Bolland M, Benn D, Croxson M, et al. Gastrointestinal stromal tumour in succinate dehydrogenase subunit B mutation-associated familial phaeochromocytoma/paraganglioma. J Surg 76(8):763–4, 2006.
Douwes Dekker PB, Hogendoorn PCW, Kuipers-Dijkshoorn N, et al. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J Pathol 201(3):480–6, 2003.
Oostveen FG, Au HC, Meijer PJ, et al. A Chinese hamster mutant cell line with a defect in the integral membrane protein CII-3 of complex II of the mitochondrial electron transport chain. Biol Chem 270(44):26104–8, 1995.
Bullis BL, Lemire BD. Isolation and characterization of the Saccharomyces cerevisiae SDH4 gene encoding a membrane anchor subunit of succinate dehydrogenase. J Biol Chem 269(9):6543–9, 1994.
Gill AJ, Chou A, Vilain R, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol 34(5):636–44, 2010.
Acknowledgments
This study was supported by grants, 2009SGR794, RD12/0036/0013, and Programa de Intensificación de la Investigación ISCIII. E.C. holds a predoctoral fellowship from AGAUR 2012FI-B2 00125. AdC is predoctoral fellows from La Caixa Fundation. Tumour samples were obtained with the support of Xarxa Catalana de Bancs de Tumours, the Tumour Banc Platform of RTICC and RD09/0076/00059, as well as the Spanish Tumour Bank Network coordinated by CNIO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castelblanco, E., Santacana, M., Valls, J. et al. Usefulness of Negative and Weak–Diffuse Pattern of SDHB Immunostaining in Assessment of SDH Mutations in Paragangliomas and Pheochromocytomas. Endocr Pathol 24, 199–205 (2013). https://doi.org/10.1007/s12022-013-9269-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-013-9269-4