Abstract
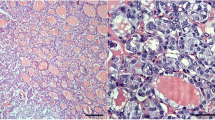
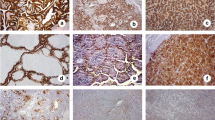
This study addressed the immunohistochemical expression of MUC1 in papillary thyroid carcinoma (PTC) of different histotypes, sizes, and morphological features of aggressiveness, and its correlation with the overexpression of cyclin D1, a target molecule of the Wnt pathway. MUC1 expression was examined in a total of 209 PTCs. Cytoplasmic MUC1 expression was elevated in the tall, columnar cell and oncocytic variants (100%), Warthin-like (78%), and conventional PTCs (61%), and in papillary microcarcinoma (PMC) with the conventional growth pattern (52%). On the contrary, it was low in the follicular variant (27%) of PTC and PMCs with follicular architecture (13%). Cytoplasmic MUC1 accumulation did not associate with any clinicopathological features except peritumoral lymphoid infiltration in PTCs and in PMCs with the conventional growth pattern. MUC1 staining correlated with cyclin D1 overexpression in conventional PTCs and PMCs and PMCs with follicular architecture. The results demonstrate that MUC1 expression varies broadly in different histological variants of PTC, being the lowest in tumors with follicular structure. In general, it does not prove to be a prognosticator of PTC aggressiveness. A high correlation between MUC1 and cyclin D1 implies MUC1 involvement in the Wnt cascade functioning in a large subset of human PTCs and PMCs.


Similar content being viewed by others
References
Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 6:339–53, 2001.
Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem 274:31751–4, 1999.
Alves P, Soares P, Fonseca E, et al. Papillary thyroid carcinoma overexpresses fully and underglycosylated mucins together with native and sialylated simple mucin antigens and histo-blood group antigens. Endocr Pathol 10:315–24, 1999.
Magro G, Schiappacassi M, Perissinotto D, et al. Differential expression of mucins 1–6 in papillary thyroid carcinoma: evidence for transformation-dependent post-translational modifications of MUC1 in situ. J Pathol 200:357–69, 2003.
Weiss M, Baruch A, Keydar I, et al. Preoperative diagnosis of thyroid papillary carcinoma by reverse transcriptase polymerase chain reaction of the MUC1 gene. Int J Cancer 66:55–9, 1996.
Bieche I, Ruffet E, Zweibaum A, et al. MUC1 mucin gene, transcripts, and protein in adenomas and papillary carcinomas of the thyroid. Thyroid 7:725–31, 1997.
Wreesmann VB, Sieczka EM, Socci ND, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res 64:3780–9, 2004.
Patel KN, Maghami E, Wreesmann VB, et al. MUC1 plays a role in tumor maintenance in aggressive thyroid carcinomas. Surgery 138:994–1001; discussion 1001–2, 2005.
Carraway KL, Ramsauer VP, Haq B, et al. Cell signaling through membrane mucins. Bioessays 25:66–71, 2003.
Schroeder JA, Adriance MC, Thompson MC, et al. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene 22:1324–32, 2003.
Wen Y, Caffrey TC, Wheelock MJ, et al. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem 278:38029–39, 2003.
Wesseling J, van der Valk SW, Vos HL, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 129:255–65, 1995.
Hatsell S, Rowlands T, Hiremath M, et al. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145–58, 2003.
Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 11:3286–305, 1997.
Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol 7:634–40, 1995.
Ishigaki K, Namba H, Nakashima M, et al. Aberrant localization of beta-catenin correlates with overexpression of its target gene in human papillary thyroid cancer. J Clin Endocrinol Metab 87:3433–40, 2002.
Nakashima M, Meirmanov S, Naruke Y, et al. Cyclin D1 overexpression in thyroid tumours from a radio-contaminated area and its correlation with Pin1 and aberrant beta-catenin expression. J Pathol 202:446–55, 2004.
Lantsov D, Meirmanov S, Nakashima M, et al. Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant beta-catenin expression. Histopathology 47:248–56, 2005.
LiVolsi V. Surgical pathology of the thyroid. Philadelphia: Saunders; 1990.
Rosai J, Carciangiu ML, DeLellis RA. Tumors of the thyroid gland. Washington, D.C.: Armed Forces Institute of Pathology; 1992.
LiVolsi VA, Albores-Saavedra J, Asa SL. Papillary carcinoma. In: DeLellis RA, Lloyd R, Hertz PhU, Eng C, editors. WHO classification of tumors. Pathology and genetics of tumors of endocrine organs. Lyon: IARC Press. p. 57–66, 2004.
Khoo ML, Ezzat S, Freeman JL, et al. Cyclin D1 protein expression predicts metastatic behavior in thyroid papillary microcarcinomas but is not associated with gene amplification. J Clin Endocrinol Metab 87:1810–3, 2002.
Hunt JL, LiVolsi VA, Baloch ZW, et al. Microscopic papillary thyroid carcinoma compared with clinical carcinomas by loss of heterozygosity mutational profile. Am J Surg Pathol 27:159–66, 2003.
Apel RL, Asa SL, LiVolsi VA. Papillary Hurthle cell carcinoma with lymphocytic stroma. “Warthin-like tumor” of the thyroid. Am J Surg Pathol 19:810–14, 1995.
Ozaki O, Ito K, Mimura T, et al. Papillary carcinoma of the thyroid. Tall-cell variant with extensive lymphocyte infiltration. Am J Surg Pathol 20:695–8, 1996.
DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 71:414–24, 1990.
Rahn JJ, Dabbagh L, Pasdar M, et al. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer 91:1973–82, 2001.
Giordano TJ, Kuick R, Thomas DG, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene 24:6646–56, 2005.
Castro P, Rebocho AP, Soares RJ, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 91:213–20, 2006.
Zhu Z, Gandhi M, Nikiforova MN, et al. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 120:71–7, 2003.
Shaha AR, Shah JP, Loree TR. Patterns of failure in differentiated carcinoma of the thyroid based on risk groups. Head Neck 20:26–30, 1998.
Acknowledgement
This work was supported in part by grant numbers 18591030, 19390253, and 19790651 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abrosimov, A., Saenko, V., Meirmanov, S. et al. The Cytoplasmic Expression of MUC1 in Papillary Thyroid Carcinoma of Different Histological Variants and its Correlation with Cyclin D1 Overexpression. Endocr Pathol 18, 68–75 (2007). https://doi.org/10.1007/s12022-007-0012-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-007-0012-x