Abstract
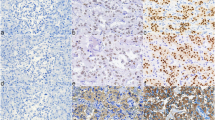
In pheochromocytomas, it is very difficult to predict malignant potential by conventional histology or immunohistochemical and molecular markers. We investigated the expression of human telomerase catalytic component (hTERT) mRNA, hTERT protein, Ki-67 antigen, and p27kip1 in pheochromocytomas (27 benign, 7 suspected malignant, and 7 malignant), and evaluated the possibility of expressions of these proteins, and hTERT mRNA serve as diagnostic markers for predicting the biological behavior of these tumors. All tumors showed the classical histology and typical immunohistochemical pattern. By in situ hybridization, hTERT mRNA was expressed in 5/7 malignant tumors (defined as the presence of metastasis and/or extensive local invasion) as compared with 3/27 benign tumors. We examined the hTERT by immunohistochemistry to confirm the mRNA. hTERT mRNA expression was correlated with hTERT protein expression. All benign tumors exhibited no immunopositivity or <1% of cells stained for Ki-67 antigen. Six out of seven malignant tumors have shown either hTERT mRNA expression or Ki-67 immunoreactivity While no statistical difference in p27kip1 expressions was observed among benign, malignant, and suspected malignant tumors, there was a statistical difference between the normal adrenal medulla samples and tumors (p<0.001). Thus, hTERT mRNA detection by in situ hybridization, hTERT expression, and Ki-67 antigen expression are all useful tools for differentiating malignant from benign pheochromocytomas.
Similar content being viewed by others

References
Nakamura TM, Cech TR. Reversing time: origin of telomerase. Cell 92: 587–590, 1998.
Meyerson M. Role of relomerase in normal and cancer cells. J Clin Oncol 18: 2626–2634, 2000.
Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015, 1994.
Tang SJ, Dumot JA, Wang L, et al. Telomerase activity in pancreatic endocrine tumors. Am J Gastroenterol 97(4):1022–1030, 2002.
Umbricht CB, Conrad GT, Clark DP, et al. Human telomerase reverse transcriptase gene expression and the surgical management of suspicious thyroid tumors. Clin Cancer Res 10:5762–5768, 2004.
Orlando C, Gelmini S. Telomerase in endocrine and endocrine-dependent tumors. J Steroid Biochem Mol Biol 78(3):201–214, 2001.
Michalides RJ. Cell cycle regulators: mechanisms and their role in aetiology, prognosis, and treatment of cancer. J Clin Pathol 52:555–568, 1999.
Hommura F, Dosaka-Akita H, Mishina T, et al. Prognostic significance of p27KIP1 protein and Ki-67 growth fraction in non-small cell lung cancers. Clin Cancer Res 6:4073–4081, 2000.
Manne U, Jhala NC, Jones J, et al. Prognostic significance of p27kip-1 expression in colorectal adenocarcinomas is associated with tumor stage. Clin Cancer Res 10:1743–1752, 2004.
Schrantz N, Beney GE, Auffredou MT, et al. The expression of p18INK4 and p27kip1 cyclin-dependent kinase inhibitors is regulated differently during human B cell differentiation. J Immunol 165:4346–4352, 2000.
Motti ML, Califano D, Troncone G, et al. Complex regulation of the cyclin-dependent kinase inhbitor p27kipl in thyroid cancer cells by the PI3K/AKT pathway: regulation of p27kipl expression and localization. Am J Pathol 166:737–749, 2005.
Lloyd RV, Jin L, Qian X, et al. Aberrant p27kipl expression in endocrine and other tumors. Am J Pathol 150:401–407, 1997.
Pace V, Pharmates E, Germann PG. Pheochromocytomas and ganglioneuromas in the aging rats: morphological and immunohistochemical characterization. Toxicol Pathol 30(4):492–500, 2002.
Isobe K, Yashiro T, Omura S, et al. Expression of the human telomerase reverse transcriptase in pheochromocytoma and neuroblastoma tissues. Endocr J 51(1):47–52, 2004.
Elder EE, Xu D, Hoog A, et al. KI-67 and hTERT expression can aid in the distinction between malignant and benign pheochromocytoma and paraganglioma. Mod Pathol 16(3):246–255, 2003.
Thompson LD. Pheochromocytoma of the adrenal gland scaled score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol 26(5):551–566, 2002.
Linnoila RI, Keiser HR, Steinberg SM, et al. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol 21:1168–1180, 1990.
van der Harst E, Bruining HA, Jaap Bonjer H, et al. Proliferative index in phaeochromocytomas: does it predict the occurrence of metastases? J Pathol 191:175–180, 2000.
Kimura N, Watanabe T, Noshiro T, et al. Histological grading of adrenal and extra-adrenal pheochromocytomas and relationship to prognosis: a clinicopathological analysis of 116 adrenal pheochromocytomas and 30 extradrenal sympathetic paragangliomas including 38 malignant tumors. Endocr Pathol 16(1):23–32, 2005.
Nakamura TM, Morin GB, Chapman KB, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955–959, 1997.
Hadar T, Shvero J, Yaniv E, et al. Expression of p53, Ki-67 and Bcl-2 in parathyroid adenoma and residual normal tissue. Pathol. Oncol Res. 11(1):45–49, 2005.
Bravaccini S, Sanchin MA, Amadori A, et al. Potential of telomerase expression and activity in cervical specimens as a diagnostic tool. J Clin Pathol 58(9):911–914, 2005.
Culhaci N, Sagol O, Karademir S, et al. Expression of transforming growth factor beta-1 and p27Kipl in pancreatic adenocarcinomas: relation with cell-cycle-associated proteins and clinicopathologic characteristics. BMC Cancer 5:98, 2005.
Catzavelos C, Bhattacharya N, Ung YC, et al. Decreased levels of the cell-cycle inhibitor p27Kipl protein: prognostic implications in primary breast cancer. Nat Med 3:227–230, 1997.
Porter PL, Malone KE, Heagerty PJ, et al. Expression of cell-cycle regulators p27Kipl and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 3:222–225, 1997.
Horikawa I, Barrett JC. Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms. Carcinogenesis 24(7):1167–1176, 2003.
Hiyama E, Hiyama K, Yokoyama T, et al. Correlating relomerase activity levels with human neuroblastoma outcomes. Nat Med 1:249–255, 1995.
Boltze C, Mundschenk J, Unger N, et al. Expression profile of the telomeric complex discriminates between benign and malignant pheochromocytoma. J Clin Endocrinol Metabol 88(9):4280–4286, 2003.
Kubota Y, Nakada T, Sasagawa I, et al. Elevated levels of telomerase activity in malignant pheochromocytoma, Cancer 82:176–179, 1998.
Bamberger CM, Else T, Bamberger AM, et al. Telomerase activity in benign and malignant adrenal tumors. Exp Clin Endocrinol Diabetes 107:272–275, 1999.
Isola JJ, Helin HJ, Helle MJ, et al. Evaluation of cell proliferation in breast carcinoma. Comparison of Ki-67 immunohistochemical study, DNA flow cytometric analysis and mitotic count. Cancer 65:1180–1184, 1990.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Z., Li, J., Qin, Y. et al. Differential expression of human telomerase catalytic subunit mRNA by In situ hybridization in pheochromocytomas. Endocr Pathol 17, 387–398 (2006). https://doi.org/10.1007/s12022-006-0010-4
Issue Date:
DOI: https://doi.org/10.1007/s12022-006-0010-4