Abstract
Purpose
Innate immune components participate in obesity-induced inflammation, which can contribute to endocrine dysfunction during metabolic diseases. However, the chronological activation of specific immune proteins such as Nucleotide-binding oligomerization domain-containing protein 1 (NOD1) and relevance to cellular crosstalk during the progression of obesity-associated insulin resistance (IR) is not known.
Methods
The NOD1 signaling in various insulin-sensitive metabolic tissues during the progression of diet-insulin resistance was assessed in C57BL/6J mice fed with 60% high-fat diet (HFD) for 4, 8, 12, and 16 weeks. Intestinal permeability was measured using FITC-dextran. NOD1 activating potential was analyzed using HEK-Blue mNOD1 cells.
Results
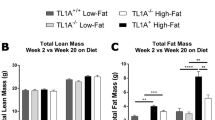
HFD-fed mice showed progressive induction of glucose intolerance and impairment of insulin signaling in key metabolic tissues. We found a time-dependent increase in intestinal permeability coupled with transport and accumulation of NOD1 activating ligand in the serum of HFD-fed mice. We also observed a progressive accumulation of γ-D-glutamyl-meso-diaminopimelic acid (DAP), a microbial peptidoglycan ligand known to activate NOD1, in serum samples of the HFD-fed mice. There was also a progressive increase in transcripts levels of NOD1 in bone marrow-derived macrophages during HFD-feeding. In addition, skeletal muscle, adipose and liver, the key insulin sensitive metabolic tissues also had a time-dependent increase in transcripts of NOD1 and Rip2 and a corresponding activation of pro-inflammatory responses in these tissues.
Conclusion
These data highlight the correlation of inflammation and insulin resistance to NOD1 activation in the bone marrow derived macrophages and insulin responsive metabolic tissues during high fat diet feeding in mice.






Similar content being viewed by others
Availability of data and materials
Data supporting the reported results will be available with the corresponding author (AK Tamrakar).
References
F.B. Hu, A. Satija, J.E. Manson, Curbing the diabetes pandemic: the need for global policy solutions. JAMA 313, 2319–2320 (2015)
J.M. Olefsky, C.K. Glass, Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010)
S. Schenk, M. Saberi, J.M. Olefsky, Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest. 118, 2992–3002 (2008)
R. Medzhitov, Inflammation 2010: new adventures of an old flame. Cell 140, 771–776 (2010)
G.S. Hotamisligil, Inflammation and metabolic disorders. Nature 444, 860–867 (2006)
N. Fei, L. Zhao, An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 7, 880–884 (2013)
P.D. Cani, R. Bibiloni, C. Knauf, A. Waget, A.M. Neyrinck, N.M. Delzenne et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008)
Y.Y. Lam, C.W.Y. Ha, C.R. Campbell, A.J. Mitchell, A. Dinudom, J. Oscarsson et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 7, e34233 (2012)
P.D. Cani, J. Amar, M.A. Iglesias, M. Poggi, C. Knauf, D. Bastelica et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007)
J. Amar, R. Burcelin, J.B. Ruidavets, P.D. Cani, J. Fauvel, M.C. Alessi, Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 87, 1219–1223 (2008)
S. Pendyala, J.M. Walker, P.R. Holt, A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142, 1100–1101.e2 (2012)
J.B. Grigg, G.F. Sonnenberg, Host-microbiota interactions shape local and systemic inflammatory diseases. J. Immunol. 198, 564–571 (2017)
F.F. Anhe, B.A.H. Jensen, T.V. Varin, F. Servant, S.V. Blerk, D. Richard et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2, 233–242 (2020)
J.K. Nicholson, E. Holmes, J. Kinross, R. Burcelin, G. Gibson, W. Jia et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012)
W.J. Lee, K. Hase, Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 10, 416–424 (2014)
K.L. Alexander, S.R. Targan, C.O. Elson, 3rd Microbiota activation and regulation of innate and adaptive immunity. Immunol. Rev. 260, 206–220 (2014)
W. Chi, D. Dao, T.C. Lau, B.D. Henriksbo, J.F. Cavallari, K.P. Foley et al. Bacterial peptidoglycan stimulates adipocyte lipolysis via NOD1. PLoS ONE 9, e97675 (2014)
H. Shi, M.V. Kokoeva, K. Inouye, I. Tzameli, H. Yin, J.S. Flier, TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006)
T.B. Clarke, K.M. Davis, E.S. Lysenko, A.Y. Zhou, Y. Yu, J.N. Weiser, Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 (2010)
J.D. Schertzer, A.K. Tamrakar, J.G. Magalhães, S. Pereira, P.J. Bilan, M.D. Fullerton et al. NOD1 activators link innate immunity to insulin resistance. Diabetes 60, 2206–2215 (2011)
J.F. Cavallari, M.D. Fullerton, B.M. Duggan, K.P. Foley, E. Denou, B.K. Smith et al. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab. 25, 1063–1074 e1063 (2017)
M. Hedl, J. Li, J.H. Cho, C. Abraham, Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc. Natl Acad. Sci. USA 104, 19440–19445 (2007)
E. Denou, K. Lolmède, L. Garidou, C. Pomie, C. Chabo, T.C. Lau et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol. Med. 7, 259–274 (2015)
J.F. Cavallari, N.T. Pokrajac, S. Zlitni, K.P. Foley, B.D. Henriksbo, J.D. Schertzer, NOD2 in hepatocytes engages a liver-gut axis to protect against steatosis, fibrosis, and gut dysbiosis during fatty liver disease in mice. Am. J. Physiol. Endocrinol. Metab. 319, E305–E314 (2020)
Z. Huang, J. Wang, X. Xu, H. Wang, Y. Qiao, W.C. Chu et al. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat. Microbiol. 4, 766–773 (2019)
J. Amar, C. Chabo, A. Waget, P. Klopp, C. Vachoux, L.G. Bermúdez-Humarán et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 3, 559–572 (2011)
K.L. Chan, T.H. Tam, P. Boroumand, D. Prescott, S.R. Costford, N.K. Escalante et al. Circulating NOD1 activators and hematopoietic NOD1 contribute to metabolic inflammation and insulin resistance. Cell Rep. 18, 2415–2426 (2017)
T. Masek, V. Vopalensky, P. Suchomelova, M. Pospisek, Denaturing RNA electrophoresis in TAE agarose gels. Anal. Biochem. 336, 46–50 (2005)
H. Luck, S. Tsai, J. Chung, X. Clemente-Casares, M. Ghazarian, X.S. Revelo et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 21, 527–542 (2015)
S.E. Shoelson, J. Lee, A.B. Goldfine, Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006)
J.Y. Kim, E. Omori, K. Matsumoto, G. Nunez, J. Ninomiya-Tsuji, TAK1 is a central mediator of NOD2 signaling in epidermal cells. J. Biol. Chem. 283, 137–144 (2008)
T. Stroh, A. Batra, R. Glauben, I. Fedke, U. Erben, A. Kroesen, Nucleotide oligomerization domain 1 and 2: regulation of expression and function in preadipocytes. J. Immunol. 181, 3620–3627 (2008)
A.K. Tamrakar, J.D. Shertzer, T.T. Chiu, K.P. Foley, P.J. Bilan, D.J. Philpott et al. NOD2 activation induces muscle cell-autonomous innate immune responses and insulin resistance. Endocrinology 151, 5624–5637 (2010)
C.K. Maurya, D. Arha, A.K. Rai, S.K. Kumar, J. Pandey, D.R. Avisetti, NOD2 activation induces oxidative stress contributing to mitochondrial dysfunction and insulin resistance in skeletal muscle cells. Free Radic. Biol. Med. 89, 158–169 (2015)
A. Sharma, C.K. Maurya, D. Arha, A.K. Rai, S. Singh, S. Varshney et al. Nod1-mediated lipolysis promotes diacylglycerol accumulation and successive inflammation via PKCδ-IRAK axis in adipocytes. Biochim. Biophys. Acta Mol. Basis Dis. 136–146, 2019 (1865)
S.E. Girardin, I.G. Boneca, L.A. Carneiro, A. Antignac, M. Jéhanno, J. Viala et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003)
M. Chamaillard, M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4, 702–707 (2003)
J.B. McPhee, J.D. Schertzer, Immunometabolism of obesity and diabetes: microbiota link compartmentalized immunity in the gut to metabolic tissue inflammation. Clin. Sci. (Lond.) 129, 1083–1096 (2015)
P.J. Turnbaugh, F. Bäckhed, L. Fulton, J.I. Gordon, Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008)
B.O. Schroeder, F. Bäckhed, Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089 (2016)
K.A. Cloud-Hansen, S.B. Peterson, E.V. Stabb, W.E. Goldman, M.J. McFall-Ngai, J. Handelsman, Breaching the great wall: peptidoglycan and microbial interactions. Nat. Rev. Microbiol. 4, 710–716 (2006)
J.W. Johnson, J.F. Fisher, S. Mobashery, Bacterial cell-wall recycling. Ann. N. Y. Acad. Sci. 1277, 54–75 (2013)
Z. Huang, J. Wang, X. Xu, H. Wang, Y. Qiao, W.C. Chu, S. Xu et al. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat. Microbiol. 4, 766–773 (2019)
A.J. Wolf, D.M. Underhill, Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 18, 243–254 (2018)
M.J. Lappas, NOD1 expression is increased in the adipose tissue of women with gestational diabetes. Endocrinol 222, 99–112 (2014)
Y.J. Zhou, C. Liu, C.L. Li, Y.L. Song, Y.S. Tang, H. Zhou et al. Increased NOD1, but not NOD2, activity in subcutaneous adipose tissue from patients with metabolic syndrome. Obes. (Silver Spring) 23, 1394–400 (2015)
R.A. DeFronzo, D. Tripathy, Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, S157–S163 (2009)
C.M. Taniguchi, B. Emanuelli, R.C. Kahn, Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006)
R. Medzhitov, Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007)
L. Zhao, M. Kwon, S. Huang, J.Y. Lee, K. Fukase, N. Inohara et al. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J. Biol. Chem. 282, 11618–11628 (2007)
Funding
This work was supported by grant from the Department of Biotechnology, New Delhi, India [No. BT/PR15667/BRB/10/1465/2015]. AS, SS, SA and FG are supported by Research Fellowship from the Council of Scientific and Industrial Research (CSIR) New Delhi. JDS holds a grant from the Canadian Institutes of Health Research (CIHR; FDN -154295) and a Canada Research Chair in Metabolic Inflammation.
Author information
Authors and Affiliations
Contributions
AS, SS, AM, and AKR conducted the experiments, analyzed the data and drafted the manuscript. IA, SA and FG contributed to acquisition and analysis of data. JDS and AS interpreted the data and reviewed and edited the manuscript. AKT contributed to the design and analysis of the study, interpreted the data, wrote and reviewed the manuscript. All listed authors approved the final version of the manuscript. This manuscript bears the CDRI communication No. 10351.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Animal study reported in the manuscript was approved by Institutional Animal Ethics Committee (IAEC) of the CSIR-Central Drug Research Institute, Lucknow.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sharma, A., Singh, S., Mishra, A. et al. Insulin resistance corresponds with a progressive increase in NOD1 in high fat diet-fed mice. Endocrine 76, 282–293 (2022). https://doi.org/10.1007/s12020-022-02995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-02995-z