Abstract
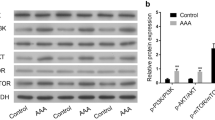
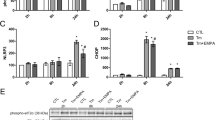
Endoplasmic reticulum stress (ERS) is involved in the development of abdominal aortic aneurysm (AAA). Since bioactive peptide intermedin (IMD)1-53 protects against AAA formation, here we investigated whether IMD1–53 attenuates AAA by inhibiting ERS. AAA model was induced by angiotensin II (AngII) in ApoE KO mouse background. AngII-treated mouse aortas showed increased ERS gene transcription of caspase12, eukaryotic translation initiation factor 2a (eIf2a) and activating transcription factor 4(ATF4).The protein level of ERS marker glucose regulated protein 94(GRP94), ATF4 and C/EBP homologous protein 10(CHOP) was also up-regulated by AngII. Increased ERS levels were accompanied by severe VSMC apoptosis in human AAA aorta. In vivo administration of IMD1-53 greatly reduced AngII-induced AAA and abrogated the activation of ERS. To determine whether IMD inhibited AAA by ameliorating ERS, we used 2 non-selective ERS inhibitors phenyl butyrate (4-PBA) and taurine (TAU). Similar to IMD, PBA, and TAU significantly reduced the incidence of AAA and AAA-related pathological disorders. In vitro, AngII infusion up-regulated CHOP, caspase12 expression and led to VSMC apoptosis. IMD siRNA aggravated the CHOP, caspase12-mediated VSMC apoptosis, which was abolished by ATF4 silencing. IMD infusion promoted the phosphorylation of adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) in aortas in ApoE KO mice, and the AMPK inhibitor compound C abolished the protective effect of IMD on VSMC ERS and apoptosis induced by AngII. In conclusion, IMD may protect against AAA formation by inhibiting ERS via activating AMPK phosphorylation.







Similar content being viewed by others
References
F.L. Moll, J.T. Powell, G. Fraedrich, F. Verzini, S. Haulon, M. Waltham, J.A. van Herwaarden, P.J. Holt, J.W. van Keulen, B. Rantner, F.J. Schlosser, F. Setacci, J.B. Ricco; S. European Society for Vascular, Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 41(Suppl 1), S1–S58 (2011)
K. Sugamura, J.F. Keaney Jr., Nicotine: linking smoking to abdominal aneurysms. Nat. Med. 18, 856–858 (2012)
F.A. Lederle, S.E. Wilson, G.R. Johnson, D.B. Reinke, F.N. Littooy, C.W. Acher, D.J. Ballard, L.M. Messina, I.L. Gordon, E.P. Chute, W.C. Krupski, S.J. Busuttil, G.W. Barone, S. Sparks, L.M. Graham, J.H. Rapp, M.S. Makaroun, G.L. Moneta, R.A. Cambria, R.G. Makhoul, D. Eton, H.J. Ansel, J.A. Freischlag, D. Bandyk; D. Aneurysm, G. Management Veterans Affairs Cooperative Study, Immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 346, 1437–1444 (2002)
F.M. Davis, D.L. Rateri, A. Daugherty, Mechanisms of aortic aneurysm formation: translating preclinical studies into clinical therapies. Heart 100, 1498–1505 (2014)
M.E. Lindsay, H.C. Dietz, Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473, 308–316 (2011)
T. Obama, T. Tsuji, T. Kobayashi, Y. Fukuda, T. Takayanagi, Y. Taro, T. Kawai, S.J. Forrester, K.J. Elliott, E. Choi, A. Daugherty, V. Rizzo, S. Eguchi, Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin. Sci. 128, 559–565 (2015)
A. Lopez-Candales, D.R. Holmes, S. Liao, M.J. Scott, S.A. Wickline, R.W. Thompson, Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathol. 150, 993–1007 (1997)
T. Minamino, I. Komuro, M. Kitakaze, Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 107, 1071–1082 (2010)
P. Walter, D. Ron, The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011)
D. Ron, P. Walter, Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007)
T. Takayanagi, K.J. Crawford, T. Kobayashi, T. Obama, T. Tsuji, K.J. Elliott, T. Hashimoto, V. Rizzo, S. Eguchi, Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin. Sci. 126, 785–794 (2014)
J. Roh, C.L. Chang, A. Bhalla, C. Klein, S.Y. Hsu, Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J. Biol. Chem. 279, 7264–7274 (2004)
Y. Takei, K. Inoue, M. Ogoshi, T. Kawahara, H. Bannai, S. Miyano, Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 556, 53–58 (2004)
J.H. Yang, Y.X. Jia, C.S. Pan, J. Zhao, M. Ouyang, J. Yang, J.K. Chang, C.S. Tang, Y.F. Qi, Effects of intermedin(1-53) on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem. Biophys. Res. Commun. 327, 713–719 (2005)
Y. Hong, D.L. Hay, R. Quirion, D.R. Poyner, The pharmacology of adrenomedullin 2/intermedin. Br. J. Pharmacol. 166, 110–120 (2012)
L. Jolly, J.E. March, P.A. Kemp, T. Bennett, S.M. Gardiner, Mechanisms involved in the regional haemodynamic effects of intermedin (adrenomedullin 2) compared with adrenomedullin in conscious rats. Br. J. Pharmacol. 157, 1502–1513 (2009)
J.R. Chang, X.H. Duan, B.H. Zhang, X. Teng, Y.B. Zhou, Y. Liu, Y.R. Yu, Y. Zhu, C.S. Tang, Y.F. Qi, Intermedin1-53 attenuates vascular smooth muscle cell calcification by inhibiting endoplasmic reticulum stress via cyclic adenosine monophosphate/protein kinase A pathway. Exp. Biol. Med. 238, 1136–1146 (2013)
W.W. Lu, L. Zhao, J.S. Zhang, Y.L. Hou, Y.R. Yu, M.Z. Jia, C.S. Tang, Y.F. Qi, Intermedin1-53 protects against cardiac hypertrophy by inhibiting endoplasmic reticulum stress via activating AMP-activated protein kinase. J. Hypertens. 33, 1676–1687 (2015)
W.W. Lu , L.X. Jia , X.Q. Ni , L. Zhao , J.S. Chang , J.S. Zhang , Y.L. Hou , Y. Zhu , Y.F. Guan , Y.R. Yu , J. Du , C.S. Tang , Y.F. Qi , , Intermedin1-53 attenuates abdominal aortic aneurysm by inhibiting oxidative stress. Arterioscler. Thromb. Vasc. Biol. 36, 2176–2190 (2016).
K. Satoh, P. Nigro, T. Matoba, M.R. O’Dell, Z. Cui, X. Shi, A. Mohan, C. Yan, J. Abe, K.A. Illig, B.C. Berk, Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat. Med. 15, 649–656 (2009)
X.Y. Dai, Y. Cai, W. Sun, Y. Ding, W. Wang, W. Kong, C. Tang, Y. Zhu, M.J. Xu, X. Wang, Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA. Cardiovasc. Res. 101, 297–305 (2014)
C. Trapnell, A. Roberts, L. Goff, G. Pertea, D. Kim, D.R. Kelley, H. Pimentel, S.L. Salzberg, J.L. Rinn, L. Pachter, Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012)
W. Huang da, B.T. Sherman, R.A. Lempicki, Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009)
J.R. Chang, J. Guo, Y. Wang, Y.L. Hou, W.W. Lu, J.S. Zhang, Y.R. Yu, M.J. Xu, X.Y. Liu, X.J. Wang, Y.F. Guan, Y. Zhu, J. Du, C.S. Tang, Y.F. Qi, Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of alpha-Klotho. Kidney Int. 89, 586–600 (2016)
X. Teng, J. Song, G. Zhang, Y. Cai, F. Yuan, J. Du, C. Tang, Y. Qi, Inhibition of endoplasmic reticulum stress by intermedin(1-53) protects against myocardial injury through a PI3 kinase-Akt signaling pathway. J. Mol. Med. 89, 1195–1205 (2011)
E. Szegezdi, S.E. Logue, A.M. Gorman, A. Samali, Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7, 880–885 (2006)
X.H. Duan, J.R. Chang, J. Zhang, B.H. Zhang, Y.L. Li, X. Teng, Y. Zhu, J. Du, C.S. Tang, Y.F. Qi, Activating transcription factor 4 is involved in endoplasmic reticulum stress-mediated apoptosis contributing to vascular calcification. Apoptosis 18, 1132–1144 (2013)
Y. Cai, L. Yang, G. Hu, X. Chen, F. Niu, L. Yuan, H. Liu, H. Xiong, J. Arikkath, S. Buch, Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy. J. Cell Biol. 215, 245–258 (2016)
Z. Wang, J. Yan, L. Li, N. Liu, Y. Liang, W. Yuan, X. Chen, Effects of Nepsilon-carboxymethyl-Lysine on ERS-mediated apoptosis in diabetic atherosclerosis. Int. J. Cardiol. 172, e478–e483 (2014)
J. Wang, X. Hu, H. Jiang, ERS-PERK signaling pathway-mediated Nrf2/ARE-HO-1 axis: A novel therapeutic target for attenuating myocardial ischemia and reperfusion injury. Int. J. Cardiol. 203, 779–780 (2016)
A.H. Hasty, D.G. Harrison, Endoplasmic reticulum stress and hypertension-a new paradigm? J. Clin. Invest. 122, 3859–3861 (2012)
P. Dromparis, R. Paulin, T.H. Stenson, A. Haromy, G. Sutendra, E.D. Michelakis, Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation 127, 115–125 (2013)
J.H. Lin, P. Walter, T.S. Yen, Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 3, 399–425 (2008)
H. Tsukano, T. Gotoh, M. Endo, K. Miyata, H. Tazume, T. Kadomatsu, M. Yano, T. Iwawaki, K. Kohno, K. Araki, H. Mizuta, Y. Oike, The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 30, 1925–1932 (2010)
Y. Wang, H. Ait-Oufella, O. Herbin, P. Bonnin, B. Ramkhelawon, S. Taleb, J. Huang, G. Offenstadt, C. Combadiere, L. Renia, J.L. Johnson, P.L. Tharaux, A. Tedgui, Z. Mallat, TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 120, 422–432 (2010)
A.K. Sharma, G. Lu, A. Jester, W.F. Johnston, Y. Zhao, V.A. Hajzus, M.R. Saadatzadeh, G. Su, C.M. Bhamidipati, G.S. Mehta, I.L. Kron, V.E. Laubach, M.P. Murphy, G. Ailawadi, G.R. Upchurch Jr., Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 126, S38–S45 (2012)
J. Zhang, J. Sun, J.S. Lindholt, G.K. Sukhova, M. Sinnamon, R.L. Stevens, R. Adachi, P. Libby, R.W. Thompson, G.P. Shi, Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ. Res. 108, 1316–1327 (2011)
X. Ni, J. Zhang, C. Tang, Y. Qi, Intermedin/adrenomedullin2: an autocrine/paracrine factor in vascular homeostasis and disease. Sci. China Life Sci. 57, 781–789 (2014)
X. Zhang, L. Gu, X. Chen, S. Wang, X. Deng, K. Liu, Z. Lv, R. Yang, S. He, Y. Peng, D. Huang, W. Jiang, K. Wu, Intermedin ameliorates atherosclerosis in ApoE null mice by modifying lipid profiles. Peptides 37, 189–193 (2012)
X.Y. Dai, Y. Cai, D.D. Mao, Y.F. Qi, C. Tang, Q. Xu, Y. Zhu, M.J. Xu, X. Wang, Increased stability of phosphatase and tensin homolog by intermedin leading to scavenger receptor A inhibition of macrophages reduces atherosclerosis in apolipoprotein E-deficient mice. J. Mol. Cell. Cardiol. 53, 509–520 (2012)
X. Qiao, R.S. Li, H. Li, G.Z. Zhu, X.G. Huang, S. Shao, B. Bai, Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am. J. Physiol. Ren. Physiol. 304, F112–F119 (2013)
S.M. Yang, J. Liu, C.X. Li, Intermedin protects against myocardial ischemia-reperfusion injury in hyperlipidemia rats. Genet. Mol. Res. 13, 8309–8319 (2014)
K. Liu, X. Deng, L. Gong, X. Chen, S. Wang, H. Chen, X. Chen, B. Amrit, S. He, The effect of intermedin on angiotensin II and endothelin-1 induced ventricular myocyte hypertrophy in neonatal rat. Clin. Lab. 59, 589–596 (2013)
S.Z. Mao, X.F. Fan, F. Xue, R. Chen, X.Y. Chen, G.S. Yuan, L.G. Hu, S.F. Liu, Y.S. Gong, Intermedin modulates hypoxic pulmonary vascular remodeling by inhibiting pulmonary artery smooth muscle cell proliferation. Pulm. Pharmacol. Ther. 27, 1–9 (2014)
J.S. Zhang, Y.L. Hou, W.W. Lu, X.Q. Ni, F. Lin, Y.R. Yu, C.S. Tang, Y.F. Qi, Intermedin protects against myocardial fibrosis by inhibiting endoplasmic reticulum stress and inflammation induced by homocysteine in apolipoprotein E-deficient mice. J. Atheroscler. Thromb. 23, 1294–1306 (2016).
Y. Wang, J. Tian, X. Qiao, X. Su, Y. Mi, R. Zhang, R. Li, Intermedin protects against renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress. BMC Nephrol. 16, 169 (2015)
R.W. Thompson, S. Liao, J.A. Curci, Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron. Artery Dis. 8, 623–631 (1997)
J.A. Curci, Digging in the “soil” of the aorta to understand the growth of abdominal aortic aneurysms. Vascular 17(Suppl 1), S21–S29 (2009)
M. Salmon, W.F. Johnston, A. Woo, N.H. Pope, G. Su, G.R. Upchurch Jr., G.K. Owens, G. Ailawadi, KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 128, S163–S174 (2013)
D. Yamanouchi, S. Morgan, K. Kato, J. Lengfeld, F. Zhang, B. Liu, Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 30, 702–707 (2010)
M. Furmanik , C.M. Shanahan, Endoplasmic reticulum stress in arterial smooth muscle cells: a novel regulator of vascular disease. Curr. Cardiol. Rev. 13, 94–105 (2016).
R.C. Pereira, L. Stadmeyer, S.J. Marciniak, D. Ron, E. Canalis, C/EBP homologous protein is necessary for normal osteoblastic function. J. Cell. Biochem. 97, 633–640 (2006)
Z. Cai, F. Li, W. Gong, W. Liu, Q. Duan, C. Chen, L. Ni, Y. Xia, K. Cianflone, N. Dong, D.W. Wang, Endoplasmic reticulum stress participates in aortic valve calcification in hypercholesterolemic animals. Arterioscler. Thromb. Vasc. Biol. 33, 2345–2354 (2013)
T. Luo, B. Chen, X. Wang, 4-PBA prevents pressure overload-induced myocardial hypertrophy and interstitial fibrosis by attenuating endoplasmic reticulum stress. Chem. Biol. Interact. 242, 99–106 (2015)
G. Marwarha, B. Dasari, O. Ghribi, Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell. Signal. 24, 484–492 (2012)
H. Mizukami, K. Takahashi, W. Inaba, K. Tsuboi, S. Osonoi, T. Yoshida, S. Yagihashi, Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of beta-cell mass in Japanese type 2 diabetic patients. Diabetes Care 37, 1966–1974 (2014)
A. Matsuzawa, H. Nishitoh, K. Tobiume, K. Takeda, H. Ichijo, Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid. Redox Signal. 4, 415–425 (2002)
J.D. Malhotra, R.J. Kaufman, Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9, 2277–2293 (2007)
M. Thomas, D. Gavrila, M.L. McCormick, F.J. Miller Jr., A. Daugherty, L.A. Cassis, K.C. Dellsperger, N.L. Weintraub, Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation 114, 404–413 (2006)
A. Konior, A. Schramm, M. Czesnikiewicz-Guzik, T.J. Guzik, NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 20, 2794–2814 (2014)
G. Li, C. Scull, L. Ozcan, I. Tabas, NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 191, 1113–1125 (2010)
Y. Dong, M. Zhang, B. Liang, Z. Xie, Z. Zhao, S. Asfa, H.C. Choi, M.H. Zou, Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation 121, 792–803 (2010)
W.S. Cheang, X.Y. Tian, W.T. Wong, C.W. Lau, S.S. Lee, Z.Y. Chen, X. Yao, N. Wang, Y. Huang, Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5’ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arterioscler. Thromb. Vasc. Biol. 34, 830–836 (2014)
H. Kim, S.Y. Moon, J.S. Kim, C.H. Baek, M. Kim, J.Y. Min, S.K. Lee, Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am. J. Physiol. Ren. Physiol. 308, F226–F236 (2015)
D.H. Nam, J.H. Han, S. Kim, Y. Shin, J.H. Lim, H.C. Choi, C.H. Woo, Activated protein C prevents methylglyoxal-induced endoplasmic reticulum stress and cardiomyocyte apoptosis via regulation of the AMP-activated protein kinase signaling pathway. Biochem. Biophys. Res. Commun. 480, 622–628 (2016)
X.Z. Zhuo, Y. Wu, Y.J. Ni, J.H. Liu, M. Gong, X.H. Wang, F. Wei, T.Z. Wang, Z. Yuan, A.Q. Ma, P. Song, Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis 18, 800–810 (2013)
P. Sujobert, L. Poulain, E. Paubelle, F. Zylbersztejn, A. Grenier, M. Lambert, E.C. Townsend, J.M. Brusq, E. Nicodeme, J. Decrooqc, I. Nepstad, A.S. Green, J. Mondesir, M.A. Hospital, N. Jacque, A. Christodoulou, T.A. Desouza, O. Hermine, M. Foretz, B. Viollet, C. Lacombe, P. Mayeux, D.M. Weinstock, I.C. Moura, D. Bouscary, J. Tamburini, Co-activation of AMPK and mTORC1 Induces Cytotoxicity in Acute Myeloid Leukemia. Cell Rep. 11, 1446–1457 (2015)
J. Lu, Q. Wang, L. Huang, H. Dong, L. Lin, N. Lin, F. Zheng, J. Tan, Palmitate causes endoplasmic reticulum stress and apoptosis in human mesenchymal stem cells: prevention by AMPK activator. Endocrinology 153, 5275–5284 (2012)
T.W. Jung, S.Y. Lee, H.C. Hong, H.Y. Choi, H.J. Yoo, S.H. Baik, K.M. Choi, AMPK activator-mediated inhibition of endoplasmic reticulum stress ameliorates carrageenan-induced insulin resistance through the suppression of selenoprotein P in HepG2 hepatocytes. Mol. Cell. Endocrinol. 382, 66–73 (2014)
Z. Liu, L. Gan, T. Wu, F. Feng, D. Luo, H. Gu, S. Liu, C. Sun, Adiponectin reduces ER stress-induced apoptosis through PPARalpha transcriptional regulation of ATF2 in mouse adipose. Cell Death Dis. 7, e2487 (2016)
X. Dai, Y. Ding, Z. Liu, W. Zhang, M.H. Zou, Phosphorylation of CHOP (C/EBP Homologous Protein) by the AMP-Activated Protein Kinase Alpha 1 in Macrophages Promotes CHOP Degradation and Reduces Injury-Induced Neointimal Disruption In Vivo. Circ. Res. 119, 1089–1100 (2016)
Y. Pang , Y. Li , Y. Lv, L. Sun, S. Zhang, Y. Li, Y. Wang, G. Liu, M.J. Xu, X. Wang, X. Jiang, Intermedin restores hyperhomocysteinemia-induced macrophage polarization and improves insulin resistance in mice. J. Biol. Chem. 291, 12336–12345 (2016).
P. Zhang, S. Hou, J. Chen, J. Zhang, F. Lin, R. Ju, X. Cheng, X. Ma, Y. Song, Y. Zhang, M. Zhu, J. Du, Y. Lan, X. Yang, Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ. Res. 118, 388–399 (2016)
M. Martin-Alonso, A.B. Garcia-Redondo, D. Guo, E. Camafeita, F. Martinez, A. Alfranca, N. Mendez-Barbero, A. Pollan, C. Sanchez-Camacho, D.T. Denhardt, M. Seiki, J. Vazquez, M. Salaices, J.M. Redondo, D. Milewicz, A.G. Arroyo, Deficiency of MMP17/MT4-MMP proteolytic activity predisposes to aortic aneurysm in mice. Circ. Res. 117, e13–e26 (2015)
U. Raaz, A.M. Zollner, I.N. Schellinger, R. Toh, F. Nakagami, M. Brandt, F.C. Emrich, Y. Kayama, S. Eken, M. Adam, L. Maegdefessel, T. Hertel, A. Deng, A. Jagger, M. Buerke, R.L. Dalman, J.M. Spin, E. Kuhl, P.S. Tsao, Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 131, 1783–1795 (2015)
Acknowledgements
We thank Q. Shen for immunohistochemistry. This work was supported by the Major Research Plan of the National Natural Science Foundation of China for “Regulation Mechanism of Vascular Homeostasis and Remodeling” (No. 91339203 to Y.F.Q.), the National Natural Science Foundation of China (No. 81670434 and 81270407 to Y.F.Q.), the National Natural Science Foundation of China (No. 91739301 to M.H.) and the Collaborative Innovation Center for Cardiovascular Disorders (PXM2013_014226_07_000088).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
12020_2018_1657_MOESM1_ESM.tif
Supplemental figure 1 IMD treatment prevented the development of AAA. (A) AngII administration-induced AAA formation, and IMD Inhibited AAA development. Representative images of isolated mouse aortas after saline, AngII or AngII + IMD treatment for 28 days; arrows point to location of AAA. (B) Incidence of AAA formation after AngII and IMD administration. (C) Systolic blood pressure of mice at different times. Saline, n = 8; AngII, n = 8; AngII + IMD, n = 7. (D) Representative data from ultrasonography of surviving mice. Arrows, inner diameter of abdominal aorta. (E) Representative H&E staining. (F) Maximum abdominal aorta diameter of mouse aortas. (G) Verhoeff-van Gieson (VVG) staining, showing cell disarray and elastin fragmentation after AngII treatment but reversed by IMD administration. (H) Representative in situ zymography of matrix metalloproteinase (MMP) level in suprarenal aortas of ApoE−/− mice. Data are mean ± SD. * P < 0.05 compared with saline. # P < 0.05 compared with AngII
12020_2018_1657_MOESM2_ESM.tif
Supplemental figure 2 IMD treatment attenuated ERS via inhibiting NADPH oxidase. (A) Western blot analysis of protein levels of GRP94, p-PERK, ATF4, CHOP and cleaved-caspase12 in VSMCs. VSMCs were pretreated with IMD1-53 (10−7 mol/L) or DPI (10−5 mol/L) for 1 h, then stimulated with or without AngII (10−5 mol/L) for 4 h. β-actin was a control for protein loading. Results are representative of 3 experiments, and densitometry analysis is shown as ratio to β-actin. (B) Statistical analysis of A.(C)mRNA expression of NOX4 after siNOX4 or scramble siRNA treatment.(D) Western blot analysis and quantification of CHOP and cleaved-caspase12 expression in non-treated cells with or without siRNA treatment. β-actin was a control for protein loading. Data are mean ± SD. *P < 0.05 compared with scramble siRNA, #P < 0.05 compared with AngII. & P < 0.05 compared with AngII + siNOX4. n = 3 at least in each group. (E) Statistical analysis of D
12020_2018_1657_MOESM3_ESM.tif
Supplemental figure 3 IMD inhibits CHOP expression by ATF4 signaling in CRL1999 cells. (A) mRNA expression of ATF4 and IMD after siATF4, siIMD or scramble siRNA treatment. (B) Western blot analysis of protein levels of CHOP in CRL1999 cells. Cells were treated with or without siRNA. β-actin was a control for protein loading. (C) Western blot analysis and quantification of CHOP expression in cells with or without siRNA treatment. β-actin was a control for protein loading. *P < 0.05 compared with scramble siRNA, #P < 0.05 compared with AngII. $ P < 0.05 compared with AngII + siIMD. n = 3 at least in each group
Rights and permissions
About this article
Cite this article
Ni, XQ., Lu, WW., Zhang, JS. et al. Inhibition of endoplasmic reticulum stress by intermedin1-53 attenuates angiotensin II–induced abdominal aortic aneurysm in ApoE KO Mice. Endocrine 62, 90–106 (2018). https://doi.org/10.1007/s12020-018-1657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1657-6