Abstract
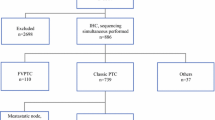
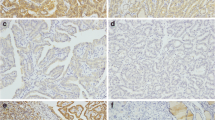
The optimal method for BRAF mutation detection remains to be determined despite advances in molecular detection techniques. The aim of this study was to compare, against classical Sanger sequencing, the diagnostic performance of two of the most recently developed, highly sensitive methods: BRAF V600E immunohistochemistry (IHC) and peptide nucleic-acid (PNA)-clamp qPCR. BRAF exon 15 mutations were searched in formalin-fixed paraffin-embedded tissues from 86 papillary thyroid carcinoma using the three methods. The limits of detection of Sanger sequencing in borderline or discordant cases were quantified by next generation sequencing. BRAF mutations were found in 74.4 % of cases by PNA, in 71 % of cases by IHC, and in 64 % of cases by Sanger sequencing. Complete concordance for the three methods was observed in 80 % of samples. Better concordance was observed with the combination of two methods, particularly PNA and IHC (59/64) (92 %), while the combination of PNA and Sanger was concordant in 55 cases (86 %). Sensitivity of the three methods was 99 % for PNA, 94.2 % for IHC, and 89.5 % for Sanger. Our data show that IHC could be used as a cost-effective, first-line method for BRAF V600E detection in daily practice, followed by PNA analysis in negative or uninterpretable cases, as the most efficient method. PNA-clamp quantitative PCR is highly sensitive and complementary to IHC as it also recognizes other mutations besides V600E and it is suitable for diagnostic purposes.




Similar content being viewed by others
Abbreviations
- PNA-clamp qPCR:
-
Nucleic acid clamp quantitative polymerase chain reaction
- IHC:
-
Immunohistochemistry
- NGS:
-
Next generation sequencing
References
D.S. Cooper, G.M. Doherty, B.R. Haugen, B.R. Hauger, R.T. Kloos, S.L. Lee, S.J. Mandel, E.L. Mazzaferri, B. McIver, F. Pacini, M. Schlumberger, S.I. Sherman, D.L. Steward, R.M. Tuttle, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009). doi:10.1089/thy.2009.0110
E.L. Mazzaferri, What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park) 23, 579–588 (2009)
Y. Ito, T. Uruno, Y. Takamura, A. Miya, K. Kobayashi, F. Matsuzuka, K. Kuma, A. Miyauchi, Papillary microcarcinomas of the thyroid with preoperatively detectable lymph node metastasis show significantly higher aggressive characteristics on immunohistochemical examination. Oncology 68, 87–96 (2005). doi:10.1159/000085701
P. Malandrino, G. Pellegriti, M. Attard, M.A. Violi, C. Giordano, L. Sciacca, C. Regalbuto, S. Squatrito, R. Vigneri, Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J. Clin. Endocrinol. Metab. 98, 1427–1434 (2013). doi:10.1210/jc.2012-3728
R.P. Tufano, G.V. Teixeira, J. Bishop, K.A. Carson, M. Xing, BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment. Medicine (Baltimore) 91, 274–286 (2012). doi:10.1097/MD.0b013e31826a9c71
C. Lupi, R. Giannini, C. Ugolini, A. Proietti, P. Berti, M. Minuto, G. Materazzi, R. Elisei, M. Santoro, P. Miccoli, F. Basolo, Extensive clinical experience: Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 92, 4085–4090 (2007). doi:10.1210/jc.2007-1179
M. Russo, P. Malandrino, M.L. Nicolosi, M. Manusia, I. Marturano, M.A. Trovato, G. Pellegriti, F. Frasca, R. Vigneri, The BRAF(V600E) mutation influences the short- and medium-term outcomes of classic papillary thyroid cancer, but is not an independent predictor of unfavorable outcome. Thyroid 24, 1267–1274 (2014). doi:10.1089/thy.2013.0675
N. Kurtulmus, M. Duren, U. Ince, M.C. Yakicier, O. Peker, O. Aydin, E. Altiok, S. Giray, H. Azizlerli, BRAFV600E mutation in Turkish patients with papillary thyroid cancer: strong correlation with indicators of tumor aggressiveness. Endocrine 42, 404–410 (2012). doi:10.1007/s12020-012-9651-x
D. Barbaro, R.M. Incensati, G. Materazzi, G. Boni, M. Grosso, E. Panicucci, P. Lapi, C. Pasquini, P. Miccoli, The BRAF V600E mutation in papillary thyroid cancer with positive or suspected pre-surgical cytological finding is not associated with advanced stages or worse prognosis. Endocrine 45, 462–468 (2014). doi:10.1007/s12020-013-0029-5
G. Gandolfi, V. Sancisi, S. Piana, A. Ciarrocchi, Time to re-consider the meaning of BRAF V600E mutation in papillary thyroid carcinoma. Int. J. Cancer 00, 1–11 (2014). doi:10.1002/ijc.28976
C. Li, K.C. Lee, E.B. Schneider, M.A. Zeiger, BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J. Clin. Endocrinol. Metab. 97, 4559–4570 (2012). doi:10.1210/jc.2012-2104
L. Fugazzola, E. Puxeddu, N. Avenia, C. Romei, V. Cirello, A. Cavaliere, P. Faviana, D. Mannavola, S. Moretti, S. Rossi, M. Sculli, V. Bottici, P. Beck-Peccoz, F. Pacini, A. Pinchera, F. Santeusanio, R. Elisei, Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr. Relat. Cancer 13, 455–464 (2006). doi:10.1677/erc.1.01086
S. Lassalle, V. Hofman, M. Ilie, C. Butori, A. Bozec, J. Santini, P. Vielh, P. Hofman, Clinical impact of the detection of BRAF mutations in thyroid pathology: potential usefulness as diagnostic, prognostic and theragnostic applications. Curr. Med. Chem. 17, 1839–1850 (2010)
G. Di Benedetto, Thyroid fine-needle aspiration: the relevance of BRAF mutation testing. Endocrine 47, 1–3 (2014). doi:10.1007/s12020-014-0222-1
J.Y. Lim, S.W. Hong, Y.S. Lee, B.-W. Kim, C.S. Park, H.-S. Chang, J.Y. Cho, Clinicopathologic implications of the BRAF(V600E) mutation in papillary thyroid cancer: a subgroup analysis of 3130 cases in a single center. Thyroid 23, 1423–1430 (2013). doi:10.1089/thy.2013.0036
P. Carbonell, M.C. Turpin, D. Torres-Moreno, I. Molina-Martínez, J. García-Solano, M. Perez-Guillermo, P. Conesa-Zamora, Comparison of allelic discrimination by dHPLC, HRM, and TaqMan in the detection of BRAF mutation V600E. J Mol Diagn 13, 467–473 (2011). doi:10.1016/j.jmoldx.2011.03.009
M.A. Ihle, J. Fassunke, K. König, I. Grünewald, M. Schlaak, N. Kreuzberg, L. Tietze, H.-U. Schildhaus, R. Büttner, S. Merkelbach-Bruse, Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p. V600E and non-p.V600E BRAF mutations. BMC Cancer 14, 13 (2014)
M. Xing, R.P. Tufano, A.P. Tufaro, S. Basaria, M. Ewertz, E. Rosenbaum, P.J. Byrne, J. Wang, D. Sidransky, P.W. Ladenson, Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J. Clin. Endocrinol. Metab. 89, 2867–2872 (2004). doi:10.1210/jc.2003-032050
A. Jarry, D. Masson, E. Cassagnau, S. Parois, C. Laboisse, M.G. Denis, Real-time allele-specific amplification for sensitive detection of the BRAF mutation V600E. Mol. Cell. Probes 18, 349–352 (2004). doi:10.1016/j.mcp.2004.05.004
Y.E. Nikiforov, N.P. Ohori, S.P. Hodak, S.E. Carty, S.O. LeBeau, R.L. Ferris, L. Yip, R.R. Seethala, M.E. Tublin, M.T. Stang, C. Coyne, J.T. Johnson, A.F. Stewart, M.N. Nikiforova, Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 96, 3390–3397 (2011). doi:10.1210/jc.2011-1469
G. He, B. Zhao, X. Zhang, R. Gong, Prognostic value of the BRAF V600E mutation in papillary thyroid carcinoma. Oncol. Lett. 7, 439–443 (2014). doi:10.3892/ol.2013.1713
R.K. Thomas, A.C. Baker, R.M. Debiasi, W. Winckler, T. Laframboise, W.M. Lin, M. Wang, W. Feng, T. Zander, L. MacConaill, L.E. Macconnaill, J.C. Lee, R. Nicoletti, C. Hatton, M. Goyette, L. Girard, K. Majmudar, L. Ziaugra, K.-K. Wong, S. Gabriel, R. Beroukhim, M. Peyton, J. Barretina, A. Dutt, C. Emery, H. Greulich, K. Shah, H. Sasaki, A. Gazdar, J. Minna, S.A. Armstrong, I.K. Mellinghoff, F.S. Hodi, G. Dranoff, P.S. Mischel, T.F. Cloughesy, S.F. Nelson, L.M. Liau, K. Mertz, M.A. Rubin, H. Moch, M. Loda, W. Catalona, J. Fletcher, S. Signoretti, F. Kaye, K.C. Anderson, G.D. Demetri, R. Dummer, S. Wagner, M. Herlyn, W.R. Sellers, M. Meyerson, L.A. Garraway, High-throughput oncogene mutation profiling in human cancer. Nat. Genet. 39, 347–351 (2007). doi:10.1038/ng1975
J. Zagzag, A. Pollack, L. Dultz, S. Dhar, J.B. Ogilvie, K.S. Heller, F.-M. Deng, K.N. Patel, Clinical utility of immunohistochemistry for the detection of the BRAF v600e mutation in papillary thyroid carcinoma. Surgery 154, 1199–1204 (2013). doi:10.1016/j.surg.2013.06.020. (discussion 1204–5)
S. Ibrahem, R. Seth, B. O’Sullivan, W. Fadhil, P. Taniere, M. Ilyas, Comparative analysis of pyrosequencing and QMC-PCR in conjunction with high resolution melting for KRAS/BRAF mutation detection. Int. J. Exp. Pathol. 91, 500–505 (2010). doi:10.1111/j.1365-2613.2010.00733.x
D.A.M.D.A.M. Heideman, I. Lurkin, M. Doeleman, E.F. Smit, H.M. Verheul, G.A. Meijer, P.J.F. Snijders, E. Thunnissen, E.C. Zwarthoff, KRAS and BRAF mutation analysis in routine molecular diagnostics: comparison of three testing methods on formalin-fixed, paraffin-embedded tumor-derived DNA. J. Mol. Diagn. 14, 247–255 (2012). doi:10.1016/j.jmoldx.2012.01.011
S.T. Lee, S.W. Kim, C.S. Ki, J.H. Jang, J.H. Shin, Y.L. Oh, J.W. Kim, J.H. Chung, Clinical implication of highly sensitive detection of the BRAF V600E mutation in fine-needle aspirations of thyroid nodules: a comparative analysis of three molecular assays in 4585 consecutive cases in a BRAF V600E mutation-prevalent area. J. Clin. Endocrinol. Metab. 97, 2299–2306 (2012). doi:10.1210/jc.2011-3135
F. Spagnolo, P. Ghiorzo, P. Queirolo, Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget 5, 10206–10221 (2014)
Y.H. Kim, S.E. Choi, S.O. Yoon, S.W. Hong, A testing algorithm for detection of the B-type Raf kinase V600E mutation in papillary thyroid carcinoma. Hum. Pathol. 45, 1483–1488 (2014). doi:10.1016/j.humpath.2014.02.025
D. Jeong, Y. Jeong, J.H. Park, S.W. Han, S.Y.S.J. Kim, Y.J. Kim, S.Y.S.J. Kim, Y. Hwangbo, S. Park, H.D. Cho, M.H. Oh, S.H. Yang, C.J. Kim, BRAF (V600E) mutation analysis in papillary thyroid carcinomas by peptide nucleic acid clamp real-time PCR. Ann. Surg. Oncol. 20, 759–766 (2013). doi:10.1245/s10434-012-2494-0
O. Koperek, C. Kornauth, D. Capper, A.S. Berghoff, R. Asari, B. Niederle, A. von Deimling, P. Birner, M. Preusser, Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am. J. Surg. Pathol. 36, 1578 (2012). doi:10.1097/PAS.0b013e3182733d9e
M.V. Pearlstein, D.C. Zedek, D.W. Ollila, A. Treece, M.L. Gulley, P.A. Groben, N.E. Thomas, Validation of the VE1 immunostain for the BRAF V600E mutation in melanoma. J. Cutan. Pathol. 41, 724–732 (2014). doi:10.1111/cup.12364
F.A. Sinicrope, T.C. Smyrk, D. Tougeron, S.N. Thibodeau, S. Singh, A. Muranyi, K. Shanmugam, T.M. Grogan, S.R. Alberts, Q. Shi, Mutation-specific antibody detects mutant BRAFV600E protein expression in human colon carcinomas. Cancer 27, 2765–2770 (2013). doi:10.1002/cncr.28133
M.I. Ilie, S. Lassalle, E. Long-Mira, C. Bonnetaud, O. Bordone, V. Lespinet, A. Lamy, J.-C. Sabourin, J. Haudebourg, C. Butori, N. Guevara, I. Peyrottes, J.-L. Sadoul, A. Bozec, J. Santini, D. Capper, A. von Deimling, J.-F. Emile, V. Hofman, P. Hofman, Diagnostic value of immunohistochemistry for the detection of the BRAF(V600E) mutation in papillary thyroid carcinoma: comparative analysis with three DNA-based assays. Thyroid 24, 858–866 (2014). doi:10.1089/thy.2013.0302
M. Bullock, C. O’Neill, A. Chou, A. Clarkson, T. Dodds, C. Toon, M. Sywak, S.B. Sidhu, L.W. Delbridge, B.G. Robinson, D.L. Learoyd, D. Capper, A. Von Deimling, R.J. Clifton-Bligh, A.J. Gill, Utilization of a MAB for BRAFV600E detection in papillary thyroid carcinoma. Endocr. Relat. Cancer 19, 779–784 (2012). doi:10.1530/ERC-12-0239
E.D. Rossi, M. Martini, S. Capodimonti, T. Cenci, P. Straccia, B. Angrisani, C. Ricci, P. Lanza, C.P. Lombardi, A. Pontecorvi, L.M. Larocca, G. Fadda, Analysis of immunocytochemical and molecular BRAF expression in thyroid carcinomas: a cytohistologic institutional experience. Cancer Cytopathol. 122, 527–535 (2014). doi:10.1002/cncy.21416
S.-R. Lee, H. Yim, J.H. Han, K.B. Lee, J. Lee, E.Y. Soh, D.J. Kim, Y.-S. Chung, S.-Y. Jeong, S.S. Sheen, S.H. Park, J.-H. Kim, VE1 antibody is not highly specific for the BRAF V600E mutation in thyroid cytology categories with the exception of malignant cases. Am. J. Clin. Pathol. 143, 437–444 (2015). doi:10.1309/AJCPOBI5CUZIBMO1
P. Ghiorzo, S. Gargiulo, L. Pastorino, S. Nasti, R. Cusano, W. Bruno, S. Gliori, M.R. Sertoli, A. Burroni, V. Savarino, F. Gensini, R. Sestini, P. Queirolo, A.M. Goldstein, G.B. Scarrà, Impact of E27X, a novel CDKN2A germ line mutation, on p16 and p14ARF expression in Italian melanoma families displaying pancreatic cancer and neuroblastoma. Hum. Mol. Genet. 15, 2682–2689 (2006). doi:10.1093/hmg/ddl199
P. Origone, S. Gargiulo, L. Mastracci, A. Ballestrero, L. Battistuzzi, C. Casella, D. Comandini, R. Cusano, A.P. Dei Tos, R. Fiocca, A. Garuti, P. Ghiorzo, C. Martinuzzi, L. Toffolatti, G. Bianchi Scarrà, Molecular characterization of an Italian series of sporadic GISTs. Gastric Cancer 16, 596–601 (2013). doi:10.1007/s10120-012-0213-y
F. Grillo, S. Pigozzi, P. Ceriolo, P. Calamaro, R. Fiocca, L. Mastracci, Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem. Cell Biol. (2015). doi:10.1007/s00418-015-1316-4
J. Collins, E.D. Rossi, A. Chandra, S.Z. Ali, Terminology and nomenclature schemes for reporting thyroid cytopathology: an overview. Semin. Diagn. Pathol. (2014). doi:10.1053/j.semdp.2014.12.007
J.Y. Seo, E.K. Kim, J.Y. Kwak, Additional BRAF mutation analysis may have additional diagnostic value in thyroid nodules with ‘suspicious for malignant’ cytology alone even when the nodules do not show suspicious US features. Endocrine 1–7, 283–289 (2014). doi:10.1007/s12020-013-0150-5
D.W. Ball, Selectively targeting mutant BRAF in thyroid cancer. J. Clin. Endocrinol. Metab. 95, 60–61 (2010). doi:10.1210/jc.2009-2332
G. Gandolfi, V. Sancisi, F. Torricelli, M. Ragazzi, A. Frasoldati, S. Piana, A. Ciarrocchi, Allele percentage of the BRAF V600E mutation in papillary thyroid carcinomas and corresponding lymph node metastases: no evidence for a role in tumor progression. J. Clin. Endocrinol. Metab. 98, 934–942 (2013). doi:10.1210/jc.2012-3930
C. Gouveia, N.T. Can, A. Bostrom, J.P. Grenert, A. van Zante, L.A. Orloff, Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: the University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg 139, 1164–1170 (2013). doi:10.1001/jamaoto.2013.4501
V. Sancisi, D. Nicoli, M. Ragazzi, S. Piana, A. Ciarrocchi, BRAFV600E mutation does not mean distant metastasis in thyroid papillary carcinomas. J. Clin. Endocrinol. Metab. 97, 1745–1749 (2012). doi:10.1210/jc.2012-1526
E. Puxeddu, S. Filetti, BRAF mutation assessment in papillary thyroid cancer: are we ready to use it in clinical practice? Endocrine 45, 341–343 (2014). doi:10.1007/s12020-013-0139-0
D. De Biase, V. Cesari, M. Visani, G.P. Casadei, N. Cremonini, G. Gandolfi, V. Sancisi, M. Ragazzi, A. Pession, A. Ciarrocchi, G. Tallini, High-sensitivity BRAF mutation analysis: bRAF V600E is acquired early during tumor development but is heterogeneously distributed in a subset of papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 99, 1530–1538 (2014). doi:10.1210/jc.2013-4389
A. Guerra, M.R. Sapio, V. Marotta, E. Campanile, S. Rossi, I. Forno, L. Fugazzola, A. Budillon, T. Moccia, G. Fenzi, M. Vitale, The primary occurrence of BRAFV600E is a rare clonal event in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 97, 517–524 (2012). doi:10.1210/jc.2011-0618
A.K. Zimmermann, U. Camenisch, M.P. Rechsteiner, B. Bode-Lesniewska, M. Rössle, Value of immunohistochemistry in the detection of BRAFV600E mutations in fine-needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol. 122, 48–58 (2014). doi:10.1002/cncy.21352
D. Capper, M. Preusser, A. Habel, F. Sahm, U. Ackermann, G. Schindler, S. Pusch, G. Mechtersheimer, H. Zentgraf, A. von Deimling, Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 122, 11–19 (2011). doi:10.1007/s00401-011-0841-z
Y.-W. Lee, Peptide nucleic acid clamp polymerase chain reaction reveals a deletion mutation of the BRAF gene in papillary thyroid carcinoma: a case report. Exp Ther Med 6, 1550–1552 (2013). doi:10.3892/etm.2013.1332
S.-H. Kang, J.Y. Pyo, S.-W. Yang, S.W. Hong, Detection of BRAF V600E mutation with thyroid tissue using pyrosequencing: comparison with PNA-clamping and real-time PCR. Am. J. Clin. Pathol. 139, 759–764 (2013). doi:10.1309/AJCPN3ULH6YWBHPH
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Grants
AIRC 15460/2014 and PRA D32I1400036005-2014 to PG.
Additional information
Paola Ghiorzo, Federica Grillo, and Luca Mastracci have shared senior contribution and are listed in alphabetical order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martinuzzi, C., Pastorino, L., Andreotti, V. et al. A combination of immunohistochemistry and molecular approaches improves highly sensitive detection of BRAF mutations in papillary thyroid cancer. Endocrine 53, 672–680 (2016). https://doi.org/10.1007/s12020-015-0720-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0720-9