Abstract
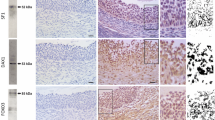
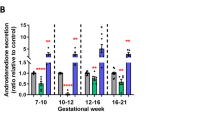
The reprograming effects of prenatal testosterone (T) treatment on postnatal reproductive parameters have been studied extensively in females of several species but similar studies in males are limited. We recently found that prenatal T treatment increases Sertoli cell number and reduced spermatogenesis in adult rams. If such disruptions are manifested early in life and involve changes in testicular paracrine environment remain to be explored. This study addresses the impact of prenatal T excess on testicular parameters in infant males, including Sertoli cell number and expression of critical genes [FSH receptor (FSHR), androgen receptor (AR), transforming growth factor beta 1 (TGFB1), 3 (TGFB3), transforming growth factor beta type 1 receptor, (TGFBR1), and anti-Müllerian hormone (AMH)] modulating testicular function. At 4 week of age, male lambs born to dams treated with 30 mg of T propionate twice weekly from day 30 to 90, followed by 40 mg of T propionate from day 90 to 120 of pregnancy (T-males), had a higher number of Sertoli cells/testis (P = 0.035) than control males (C-males) born to dams treated with the vehicle. While no differences were observed in the expression of FSHR and TGFB3, testicular TGFBR1 expression was found to be lower in T-males (P = 0.03) compared to C-males. Expression level of AMH, TGFB1, and AR also tended to be lower in T-males. These findings provide evidence that impact of fetal exposure to T excess is evident early in postnatal life, mainly characterized by an increase in Sertoli cell number. This could explain the testicular dysfunction observed in adult rams.



Similar content being viewed by others
References
S.E. Recabarren, P.P. Rojas-García, M.P. Recabarren, V.H. Alfaro, R. Smith, V. Padmanabhan, T. Sir-Petermann, Prenatal testosterone excess reduces sperm count and motility. Endocrinology 149, 6444–6448 (2008)
P.P. Rojas-García, M.P. Recabarren, L. Sarabia, J. Schön, C. Gabler, R. Einspanier, M. Maliqueo, T. Sir-Petermann, R. Rey, S.E. Recabarren, Prenatal testosterone excess alters Sertoli and germ cell number and testicular FSH receptor expression in rams. Am. J. Physiol. Endocrinol. Metab. 299, E998–E1005 (2010)
T. Sir-Petermann, M. Maliqueo, B. Angel, H.E. Lara, F. Pérez-Bravo, S.E. Recabarren, Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum. Reprod. 17, 2573–2579 (2002)
D. Tran, N. Meusy-Dessolle, N. Josso, Anti-Müllerian hormone is a functional marker of foetal Sertoli cells. Nature 269, 411–412 (1977)
R. Rey, Endocrine, paracrine and cellular regulation of postnatal anti-Mullerian hormone secretion by Sertoli cells. Trends Endocrinol. Metab. 9, 271–276 (1998)
S.E. Recabarren, T. Sir-Petermann, R. Rios, M. Maliqueo, B. Echiburú, R. Smith, P. Rojas-García, M. Recabarren, R.A. Rey, Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J. Clin. Endocrinol. Metab. 93, 3318–3324 (2008)
C. West, D.L. Foster, N.P. Evans, J. Robinson, V. Padmanabhan, Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol. Cell. Endocrinol. 185, 51–59 (2001)
S.E. Recabarren, V. Padmanabhan, E. Codner, A. Lobos, C. Durán, M. Vidal, D.L. Foster, T. Sir-Petermann, Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am. J. Physiol. Endocrinol. Metab. 289, E801–E806 (2005)
T.L. Steckler, C. Herkimer, D.A. Dumesic, V. Padmanabhan, Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity–implication for polycystic ovary syndrome. Endocrinology 150, 1456–1465 (2009)
S. Franks, Animal models and the developmental origins of polycystic ovary syndrome: increasing evidence for the role of androgens in programming reproductive and metabolic dysfunction. Endocrinology 153, 2536–2547 (2012)
B.P. Mullaney, M.K. Skinner, Transforming growth factor-beta (beta 1, beta 2, and beta 3) gene expression and action during pubertal development of the seminiferous tubule: potential role at the onset of spermatogenesis. Mol. Endocrinol. 7, 67–76 (1993)
K.J. Teerds, J.H. Dorrington, Localization of transforming growth factor beta 1 and beta 2 during testicular development in the rat. Biol. Reprod. 48, 40–45 (1993)
C. Belville, S.P. Jamin, J.Y. Picard, N. Josso, N. di Clemente, Role of type I receptors for anti-Müllerian hormone in the SMAT-1 Sertoli cell line. Oncogene 24, 4984–4992 (2005)
S. Hirobe, W.W. He, M.M. Lee, P.K. Donahoe, Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology 131, 854–862 (1992)
J. Teixeira, S. Maheswaran, P.K. Donahoe, Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr. Rev. 22, 657–674 (2001)
W.Y. Lui, W.M. Lee, C.Y. Cheng, TGF-betas: their role in testicular function and Sertoli cell tight junction dynamics. Int. J. Androl. 26, 147–160 (2003)
W.Y. Lui, W.M. Lee, C.Y. Cheng, Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology 142, 1865–1877 (2001)
W.Y. Lui, W.M. Lee, C.Y. Cheng, Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogenactivated protein kinase pathway. Biol. Reprod. 68, 1597–1612 (2003)
L. Konrad, G.H. Lüers, E. Völck-Badouin, M.M. Keilani, L. Laible, G. Aumüller, R. Hofmann, Analysis of the mRNA expression of the TGF-Beta family in testicular cells and localization of the splice variant TGF-beta2B in testis. Mol. Reprod. Dev. 73, 1211–1220 (2006)
S.E. Recabarren, P.P. Rojas-García, M.P. Recabarren, K. Norambuena, T. Sir-Petermann, Impact of prenatal exposure to testosterone in biometrics and endocrine parameters of newborn lambs. Arch. Med. Vet. 41, 43–51 (2009)
R. Rey, S. Campo, P. Bedecarrás, C. Nagle, H. Chemes, Is infancy a quiescent period of testicular development? Histological, morphometric, and functional study of the seminiferous tubules of the Cebus monkey from birth to the end of puberty. J. Clin. Endocrinol. Metab. 76, 1325–1331 (1993)
H. Mori, A.K. Christensen, Morphometric analysis of Leydig cells in the normal rat testis. J. Cell Biol. 84, 340–354 (1980)
R. Rey, C. Nagle, H. Chemes, Morphometric study of the testicular interstitial tissue of the monkey Cebus apella during postnatal development. Tissue Cell 28, 31–42 (1996)
D.J. Handelsman, S. Staraj, Testicular size: the effects of aging, malnutrition, and illness. J. Androl. 6, 144–151 (1985)
W. Muruvi, H.M. Picton, R.G. Rodway, I.M. Joyce, In vitro growth of oocytes from primordial follicles isolated from frozen–thawed lamb ovaries. Theriogenology 64, 1357–1370 (2005)
C.L. Bormann, G.D. Smith, V. Padmanabhan, T.M. Lee, Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction 142, 167–173 (2011)
M. Nistal, M.A. Abaurrea, R. Paniagua, Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J. Anat. 134, 351–363 (1982)
J. Müller, N.E. Skakkebæk, Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int. J. Androl. 6, 143–156 (1983)
C. Lukas-Croisier, C. Lasala, J. Nicaud, P. Bedecarrás, T.R. Kumar, M. Dutertre, M.M. Matzuk, J.Y. Picard, N. Josso, R. Rey, Follicle-stimulating hormone increases testicular anti-Müllerian hormone (AMH) production through Sertoli cell proliferation and a nonclassical cyclic adenosine 5′-monophosphate-mediated activation of the AMH gene. Mol. Endocrinol. 17, 550–561 (2003)
H. Johnston, P.J. Baker, M. Abel, H.M. Charlton, G. Jackson, L. Fleming, T.R. Kumar, P.J. O’Shaughnessy, Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145, 318–329 (2004)
K.A. Tan, K. De Gendt, N. Atanassova, M. Walker, R.M. Sharpe, P.T. Saunders, E. Denolet, G. Verhoeven, The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146, 2674–2683 (2005)
H.M. Scott, G.R. Hutchison, M.S. Jobling, C. McKinnell, A.J. Drake, R.M. Sharpe, Relationship between androgen action in the “male programming window,” fetal Sertoli cell number, and adult testis size in the rat. Endocrinology 149, 5280–5287 (2008)
L. You, M. Sar, Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine 9, 253–261 (1998)
G. Majdic, M.R. Millar, P.T. Saunders, Immunolocalisation of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. J. Endocrinol. 147, 285–293 (1995)
L. Al-Attar, K. Noël, M. Dutertre, C. Belville, M.G. Forest, P.S. Burgoyne, N. Josso, R. Rey, Hormonal and cellular regulation of Sertoli cell anti- Müllerian hormone production in the postnatal mouse. J. Clin. Invest. 100, 1335–1343 (1997)
E.B. Berensztein, M.S. Baquedano, C.R. Gonzalez, N.I. Saraco, J. Rodriguez, R. Ponzio, M.A. Rivarola, A. Belgorosky, Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr. Res. 60, 740–744 (2006)
H.E. Chemes, R.A. Rey, M. Nistal, J. Regadera, M. Musse, P. González-Peramato, A. Serrano, Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J. Clin. Endocrinol. Metab. 93, 4408–4412 (2008)
K. Boukari, G. Meduri, S. Brailly-Tabard, J. Guibourdenche, M.L. Ciampi, N. Massin, L. Martinerie, J.Y. Picard, R. Rey, M. Lombès, Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J. Clin. Endocrinol. Metab. 94, 1818–1825 (2009)
J.M. Orth, G.L. Gunsalus, A.A. Lamperti, Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122, 787–794 (1988)
T.A. Yarney, L.M. Sanford, Pubertal changes in the secretion of gonadotropic hormones, testicular gonadotropic receptors and testicular function in the ram. Domest. Anim. Endocrinol. 6, 219–229 (1989)
A. Wagener, J. Fickel, J. Schön, A. Fritzenkötter, F. Göritz, S. Blottner, Seasonal variation in expression and localization of testicular transforming growth factors TGF-{beta}1 and TGF-{beta}3 corresponds with spermatogenic activity in roe deer. J. Endocrinol. 187, 205–215 (2005)
Y.Q. Zhang, X.Z. He, J.S. Zhang, R.A. Wang, J. Zhou, R.J. Xu, Stage-specific localization of transforming growth factor beta1 and beta3 and their receptors during spermatogenesis in men. Asian J. Androl. 6, 105–109 (2004)
L. Su, D.D. Mruk, W.M. Lee, C.Y. Cheng, Differential effects of testosterone and TGF-B3 on endocytic vesicle-mediated protein trafficking events at the blood–testis barrier. Exp. Cell Res. 316, 2945–2960 (2010)
H.H. Yan, D.D. Mruk, W.M. Lee, C.Y. Cheng, Blood–testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 22, 1945–1959 (2008)
V. Caussanel, E. Tabone, J.C. Hendrick, F. Dacheux, M. Benahmed, Cellular distribution of transforming growth factor betas 1, 2, and 3 and their types I and II receptors during postnatal development and spermatogenesis in the boar testis. Biol. Reprod. 56, 357–367 (1997)
C. Gautier, C. Levacher, O. Avallet, M. Vigier, V. Rouiller-Fabre, L. Lecerf, J. Saez, R. Habert, Immunohistochemical localization of transforming growth factor-beta 1 in the fetal and neonatal rat testis. Mol. Cell. Endocrinol. 99, 55–61 (1994)
S.E. Recabarren, A. Lobos, Y. Figueroa, V. Padmanabhan, D.L. Foster, T. Sir-Petermann, Prenatal testosterone treatment alters LH and testosterone responsiveness to GnRH agonist in male sheep. Biol. Res. 40, 329–338 (2007)
Acknowledgments
We are grateful to Dr. José F. Cox for the facilities of the Reproductive Biotechnology Laboratory of the Universidad de Concepción; to Dr Gordon E Niswender, Leo Reichert Jr and A. F. Parlow for providing reagents for LH and FSH RIAs, to DAAD (Deutscher Akademischer Austauschdienst) for the donation of the Biophotometer. Participation of Dr Vasantha Padmanabhan and Dr Rodolfo Rey in this project was supported by International Collaboration Agreement with FONDECYT. This work was supported by Fondecyt Grant 1090031. This work was supported by the National Fund for Science and Technology Development (FONDECYT, Grant number 1090031).
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rojas-García, P.P., Recabarren, M.P., Sir-Petermann, T. et al. Altered testicular development as a consequence of increase number of sertoli cell in male lambs exposed prenatally to excess testosterone. Endocrine 43, 705–713 (2013). https://doi.org/10.1007/s12020-012-9818-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9818-5