Abstract
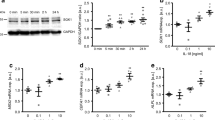
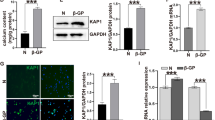
Osteopontin (OPN) is known to be one of the cytokines that is involved in the vascular inflammation caused by aldosterone (Ald). Previous reports have shown that Ald increases OPN expression, and the mechanisms for this remain to be clarified. In this study, we investigated how Ald increases OPN expression in the vascular smooth muscle cells (VSMCs) of rats. Ald increased OPN expression time dependently as well as dose dependently. This increase was diminished by spironolactone, a mineralocorticoid receptor (MR) antagonist. PD98059, an inhibitor of p42/44 MAPK pathway, and SB203580, an inhibitor of p38 MAPK pathway, suppressed Ald-induced OPN expression and secretion in VSMCs. VSMCs migration stimulated by aldosterone required OPN expression. In conclusion, these data suggest that Ald-induced OPN expression in VSMC is mediated by MR and signaling cascades involving ERK and p38 MAPK. These molecules may represent therapeutic targets for the prevention of pathological vascular remodeling.




Similar content being viewed by others
References
E.R. O’Brien, M.R. Garvin, D.K. Stewart, T. Hinohara, J.B. Simpson, S.M. Schwartz, C.M. Giachelli, Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 14, 1648–1656 (1994)
L. Liaw, M. Almeida, C.E. Hart, S.M. Schwartz, C.M. Giachelli, Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ. Res. 74, 214–224 (1994)
K. Isoda, K. Nishikawa, Y. Kamezawa, M. Yoshida, M. Kusuhara, M. Moroi, N. Tada, F. Ohsuzu, Osteopontin plays and important role in the development of medial thickening and neointimal formation. Circ. Res. 91, 77–82 (2002)
T. Cascella, Y. Radhakrishnan, L.A. Maile, W.H. Busby Jr., K. Gollahon, A. Colao, D.R. Clemmons, Aldosterone enhances IGF-I-mediated signaling and biological function in vascular smooth muscle cells. Endocrinology 151, 5851–5864 (2010)
M. Han, J.K. Wen, B. Zheng, Z. Liu, Y. Chen, Blockade of integrin β3-FAK signaling pathway activated by osteopontin inhibits neointimal formation after balloon injury. Cardiovasc. Pathol. 16, 283–290 (2007)
M. Kurata, T. Okura, S. Watanabe, T. Fukuoka, J. Higaki, Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin. Sci. (Lond.) 111, 319–324 (2006)
C.M. Giachelli, N. Bae, M. Almeida, D.T. Denhardt, C.E. Alpers, S.M. Schwartz, Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Invest. 92, 1686–1696 (1993)
R. Rocha, A.E. Rudolph, G.E. Frierdich, D.A. Nachowiak, B.K. Kekec, E.A. Blomme, E.G. McMahon, J.A. Delyani, Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 283, H1802–H1810 (2002)
D. Nagata, M. Takahashi, K. Sawai, T. Tagami, T. Usui, A. Shimatsu, Y. Hirata, M. Naruse, Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO syntheses activity. Hypertension 48, 165–171 (2006)
N. Sukor, Primary aldosteronism: from bench to bedside. Endocrine 41, 31–39 (2012)
R. Rocha, J.W. Funder, The pathophysiology of aldosterone in the cardiovascular system. Ann. N.Y. Acad. Sci. 970, 89–100 (2002)
S. Del Ry, D. Giannessi, M. Maltinti, M. Cabiati, C. Prontera, A. Iervasi, C. Caselli, A.M. Mazzone, D. Neglia, Increased plasma levels of osteopontin are associated with activation of the renin–aldosterone system and with myocardial and coronary micro-vascular damage in dilated cardiomyopathy. Cytokine 49, 325–330 (2010)
A. Kiyosue, D. Nagata, M. Myojo, T. Sato, M. Takahashi, H. Satonaka, R. Nagai, Y. Hirata, Aldosterone-induced osteopontin gene transcription in vascular smooth muscle cells involves glucocorticoid response element. Hypertens. Res. 34, 1283–1287 (2011)
T. Ishida, M. Ishida, J. Suero, M. Takahashi, B.C. Berk, Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J. Clin. Invest. 103, 789–797 (1999)
X. Jin, X. Song, L. Li, Z. Wang, Y. Tao, L. Deng, M. Tang, W. Yi, Y. Cao, Blockade of AP-1 activity by dominant-negative TAM67 can abrogate the oncogenic phenotype in latent membrane protein 1-positive human nasopharyngeal carcinoma. Mol. Carcinog. 46, 901–911 (2007)
X. Jin, X. Ge, D.L. Zhu, C. Yan, Y.F. Chu, W.D. Chen, J. Liu, P.J. Gao, Expression and function of vascular endothelial growth factor receptors (Flt-1 and Flk-1) in vascular adventitial fibroblasts. J. Mol. Cell. Cardiol. 3, 292–300 (2007)
M. Usui, K. Egashira, K. Ohtani, C. Kataoka, M. Ishibashi, K. Hiasa, M. Katoh, Q. Zhao, S. Kitamoto, A. Takeshita, Anti-monocyte chemoattractant protein-1 gene therapy inhibits restenotic changes (neointimal hyperplasia) after balloon injury in rats and monkeys. FASEB J. 16, 1838–1840 (2002)
B. Pitt, F. Zannad, W.J. Remme, R. Cody, A. Castaigne, A. Perez, J. Palensky, J. Wittes, The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N. Engl. J. Med. 341, 709–717 (1999)
B. Pitt, G. Bakris, L.M. Ruilope, L. DiCarlo, R. Mukherjee, Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 118, 1643–1650 (2008)
L. Pascual-Le Tallec, M. Lombes, The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol. Endocrinol. 19, 2211–2221 (2005)
P.J. Fuller, M.J. Young, Mechanisms of mineralocorticoid action. Hypertension 46, 1227–1235 (2005)
C. Grossmann, M. Gekle, Nongenotropic aldosterone effects and the EGFR: interaction and biological relevance. Steroids 73, 973–978 (2008)
C. Grossmann, A.W. Krug, R. Freudinger, S. Mildenberger, K. Voelker, M. Gekle, Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am. J. Physiol. Endocrinol. Metab. 292, E1790–E1800 (2007)
J. Irita, T. Okura, M. Kurata, K. Miyoshi, T. Fukuoka, J. Higaki, Osteopontin in rat renal fibroblasts: functional properties and transcriptional regulation by aldosterone. Hypertension 51, 507–513 (2008)
S. Sakurabayashi-Kitade, Y. Aoka, H. Nagashima, H. Kasanuki, N. Hagiwara, M. Kawana, Aldosterone blockade by spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis 206, 54–60 (2009)
F. Sam, Z. Xie, H. Ooi, D.L. Kerstetter, W.S. Colucci, M. Singh, K. Singh, Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am. J. Hypertens. 17, 188–193 (2004)
J. Irita, T. Okura, S. Manabe, M. Kurata, K. Miyoshi, S. Watanabe, T. Fukuoka, J. Higaki, Plasma osteopontin levels are higher in patients with primary aldosteronism than in patients with essential hypertension. Am. J. Hypertens. 19, 293–297 (2006)
H. Otani, F. Otsuka, K. Inagaki, M. Takeda, T. Miyoshi, J. Suzuki, T. Mukai, T. Ogura, H. Makino, Antagonistic effects of bone morphogenetic protein-4 and -7 on renal mesangial cell proliferation induced by aldosterone through MAPK activation. Am. J. Physiol. Renal Physiol. 292, F1513–F1525 (2007)
C. Walczak, F. Gaignier, A. Gilet, F. Zou, S.N. Thornton, A. Ropars, Aldosterone increase VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochim. Biophys. Acta 1813, 2125–2132 (2011)
Y. Zhan, S. Kim, Y. Izumi, Y. Izumiya, T. Nakao, H. Miyazaki, H. Iwao, Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration and gene expression. Arterioscler. Thromb. Vasc. Biol. 23, 795–801 (2003)
Z.H. Zhang, Y. Yu, S.G. Wei, R.B. Felder, Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am. J. Physiol. Heart Circ. Physiol. 302, H742–H751 (2012)
A. Nguyen Dinh Cat, A.M. Briones, G.E. Callera, A. Yogi, Y. He, A.C. Montezano, R.M. Touyz, Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension 58, 479–488 (2011)
K. Isoda, Y. Kamezawa, M. Ayaori, M. Kusuhara, N. Tada, F. Ohsuzu, Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation 107, 679–681 (2003)
Y. Matsui, S.R. Rittling, H. Okamoto, M. Inobe, N. Jia, T. Shimizu, M. Akino, T. Sugawara, J. Morimoto, C. Kimura, S. Kon, D. Denhardt, A. Kitabatake, T. Uede, Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 23, 1029–1034 (2003)
D. Panda, G.C. Kundu, B.I. Lee, A. Peri, D. Fohl, I. Chackalaparampil, B.B. Mukherjee, X.D. Li, D.C. Mukherjee, S. Seides, J. Rosenberg, K. Stark, A.B. Mukherjee, Potential roles of osteopontin and alphaVbeta3 integrin in the development of coronary artery restenosis after angioplasty. Proc. Natl. Acad. Sci. U.S.A. 94, 9308–9313 (1997)
J. Irita, T. Okura, M. Jotoku, T. Nagao, D. Enomoto, M. Kurata, V.R. Desilv, K. Miyoshi, Y. Matsui, T. Uede, D.T. Denhardt, S.R. Rittiling, J. Higaki, Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am. J. Physiol. Renal Physiol. 301, F833–F844 (2011)
S.M. Martin, J.L. Schwartz, C.M. Giachelli, B.D. Ratner, Enhancing the biological activity of immobilized osteopontin using a type-1 collagen affinity coating. J. Biomed. Mater. Res. A 70, 10–19 (2004)
H.E. Yim, K.H. Yoo, I.S. Bae, G.Y. Jang, Y.S. Hong, J.W. Lee, Aldosterone regulates cellular turnover and mitogen-activated protein kinase family expression in the neonatal rat kidney. J. Cell. Physiol. 219, 724–733 (2009)
Y. Nagai, K. Miyata, G.P. Sun, M. Rahman, S. Kimura, A. Miyatake, H. Kiyomoto, M. Kohno, Y. Abe, M. Yoshizumi, A. Nishiyama, Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension 46, 1039–1045 (2005)
Acknowledgments
This research was supported financially by grants from the National Basic Research Program of China (2011CB503905). We thank Miao Y for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, GX., Xu, CC., Zhong, Y. et al. Aldosterone-induced osteopontin expression in vascular smooth muscle cells involves MR, ERK, and p38 MAPK. Endocrine 42, 676–683 (2012). https://doi.org/10.1007/s12020-012-9675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9675-2