Abstract
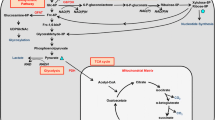
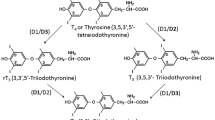
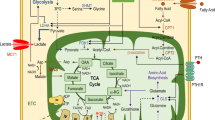
Thyroid hormones increase energy expenditure and bone turnover in vivo. To study whether 3,3′,5-triiodo-l-thyronine (T3) stimulates the uptake of glucose in osteoblastic cells, PyMS (a cell line derived from rat bone) cells were kept in serum-free culture medium and treated with T3. We measured [1-14C]-2-deoxy-d-glucose (2DG) uptake and looked for expression of the high-affinity glucose transporters GLUT1 and GLUT3 by northern and western analysis. T3 did not influence the cell number but slightly (1.3-fold) increased the protein content of the cell cultures. 2DG uptake was low in serum-deprived cell cultures and was increased by T3 (up to 2.5-fold at 1 nmol l−1 after 4 days) in a dose- and time-dependent manner. Triiodothyronine at 1 nmol l−1 increased GLUT1 and GLUT3 abundance in membranes. Therefore, increased glucose uptake induced by T3 in osteoblasts may be mediated by the known high-affinity glucose transporters GLUT1 and GLUT3.






Similar content being viewed by others
References
D.M. Thomas, F. Maher, S.D. Rogers, J.D. Best, Expression and regulation by insulin of GLUT3 in UMR 106–01, a clonal rat osteosarcoma cell line. Biochem. Biophys. Res. Commun. 218, 789–793 (1996)
E. Zoidis, C. Ghirlanda-Keller, C. Schmid, Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol. Cell. Biochem. 348, 33–42 (2011)
C. Schmid, J. Zapf, E.R. Froesch, Production of carrier proteins for insulin-like growth factors (IGFs) by rat osteoblastic cells. FEBS Lett. 244, 328–332 (1989)
C.M. Veldman, I. Schläpfer, C. Schmid, 1α,25-dihydroxyvitamin D3 stimulates sodium-dependent phosphate transport in osteoblast-like cells. Bone 21, 41–47 (1997)
C. Schmid, C. Keller, I. Schläpfer, C. Veldman, J. Zapf, Calcium and insulin-like growth factor I stimulation of sodium-dependent phosphate transport and proliferation of cultured rat osteoblasts. Biochem. Biophys. Res. Commun. 245, 220–225 (1998)
C. Schmid, C. Ghirlanda-Keller, M. Gosteli-Peter, Ascorbic acid decreases neutral endopeptidase activity in cultured osteoblastic cells. Regul. Pept. 130, 57–66 (2005)
C. Schmid, T. Steiner, E.R. Froesch, Insulin-like growth factors stimulate synthesis of nucleic acids and glycogen in cultured calvaria cells. Calcif. Tissue Int. 35, 578–585 (1983)
E.R. Froesch, C. Schmid, J. Schwander, J. Zapf, Actions of insulin-like growth factors. Annu. Rev. Physiol. 47, 443–467 (1985)
M. Ferron, J. Wei, T. Yoshizawa, A. Del Fattore, R.A. DePinho, A. Teti, P. Ducy, G. Karsenty, Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 (2010)
K. Fulzele, R.C. Riddle, D.J. DiGirolamo, X. Cao, C. Wan, D. Chen, M.-C. Faugere, S. Aja, M.A. Hussain, J.C. Brüning, T.L. Clemens, Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010)
M. López, L. Varela, M.J. Vázquez, S. Rodríguez-Cuenca, C.R. González, V.R. Velagapudi, D.A. Morgan, E. Schoenmakers, K. Agassandian, R. Lage, P.B. Martínez de Morentin, S. Tovar, R. Nogueiras, D. Carling, C. Lelliott, R. Gallego, M. Oresic, K. Chatterjee, A.K. Saha, K. Rahmouni, C. Diéguez, A. Vidal-Puig, Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 16, 1001–1008 (2010)
J.E. Silva, P.R. Larsen, Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305, 712–713 (1983)
J.E. Silva, P.R. Larsen, Potential of brown adipose tissue type II thyroxine 5′-deiodinase as a local and systemic source of triiodothyronine in rats. J. Clin. Invest. 76, 2296–2305 (1985)
S.P. Weinstein, J. Watts, P.N. Graves, R.S. Haber, Stimulation of glucose transport by thyroid hormone in ARL 15 cells: increased abundance of glucose transporter protein and messenger ribonucleic acid. Endocrinology 126, 1421–1429 (1990)
M.A. Gosteli-Peter, C. Schmid, J. Zapf, Triiodothyronine increases glucose transporter isotype 4 mRNA expression, glucose transport, and glycogen synthesis in adult rat cardiomyocytes in long-term culture. Biochem. Biophys. Res. Commun. 221, 521–524 (1996)
R. Romero, B. Casanova, N. Pulido, A.I. Suarez, E. Rodriguez, A. Rovira, Stimulation of glucose transport by thyroid hormone in 3T3-L1 adipocytes: increased abundance of GLUT1 and GLUT4 glucose transporter proteins. J. Endocrinol. 164, 187–195 (2000)
C.H.A. Gouveia, M.A. Christoffolete, C.R. Zaitune, J.M. Dora, J.W. Harney, A.L. Maia, A.C. Bianco, Type 2 iodothyronine selenodeiodinase is expressed throughout the mouse skeleton and in the MC3T3-E1 mouse osteoblastic cell line during differentiation. Endocrinology 146, 195–200 (2005)
J.H.D. Bassett, G.R. Williams, The skeletal phenotypes of TR and TRβ mutant mice. J. Mol. Endocrinol. 42, 269–282 (2009)
J.H.D. Bassett, A. Boyde, P.G.T. Howell, R.H. Bassett, T.M. Galliford, M. Archanco, H. Evans, M.A. Lawson, P. Croucher, D.L. St. Germain, V.A. Galton, G.R. Williams, Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc. Nat. Acad. Sci 107, 7604–7609 (2010)
A.I. Gogakos, J.H.D. Bassett, G.R. Williams, Thyroid and bone. Arch. Biochem. Biophys. 503, 129–136 (2010)
A. Fraichard, O. Chassande, M. Plateroti, J.P. Roux, J. Trouillas, C. Dehay, C. Legrand, K. Gauthier, M. Kedinger, L. Malaval, B. Rousset, J. Samarut, The T3Ra gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 16, 4412–4420 (1997)
S.M. Krane, G.L. Brownell, J.B. Stanbury, H. Corrigan, The effect of thyroid disease on calcium metabolism in man. J. Clin. Invest. 35, 874–887 (1956)
P. Charles, J.W. Poser, L. Mosekilde, F.T. Jensen, Estimation of bone turnover evaluated by 47calcium-kinetics: efficiency of serum bone gamma-carboxyglutamic acid-containing protein, serum alkaline phosphatase and urinary hydroxyproline excretion. J. Clin. Invest. 76, 2254–2258 (1985)
C.B. Confavreux, R.L. Levine, G. Karsenty, A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol. Cell. Endocrinol. 310, 21–29 (2009)
W.S. Simonides, M.A. Mulcahey, E.M. Redout, A. Muller, M.J. Zuidwijk, T.J. Visser, F.W. Wassen, A. Crescenzi, W.S. da-Silva, J. Harney, F.B. Engel, M.J. Obregon, P.R. Larsen, A.C. Bianco, S.A. Huang, Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J. Clin. Invest. 118, 975–983 (2008)
A. Lanni, M. Moreno, A. Lombardi, F. Goglia, Thyroid hormones and uncoupling proteins. FEBS Lett. 543, 5–10 (2003)
E. Zoidis, C. Ghirlanda-Keller, M. Gosteli-Peter, J. Zapf, C. Schmid, Regulation of phosphate (Pi) transport and NaPi-III transporter (Pit-1) mRNA in rat osteoblasts. J. Endocrinol. 181, 531–540 (2004)
C. Schmid, I. Schlapfer, M. Peter, M. Boni-Schnetzler, J. Schwander, J. Zapf, E.R. Froesch, Growth hormone and parathyroid hormone stimulate IGFBP-3 in rat osteoblasts. Am. J. Physiol. 267, 226–233 (1994)
Zorzano, A., Palacín, M., Gumà, A.: Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol. Scand. 183, 43–58 (2005)
V. Lebon, S. Dufour, K.F. Petersen, J. Ren, B.M. Jucker, L.A. Slezak, G.W. Cline, D.L. Rothman, G.I. Shulman, Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J. Clin. Invest. 108, 733–737 (2001)
K. Clement, N. Viguerie, M. Diehn, A. Alizadeh, P. Barbe, C. Thalamas, J.D. Storey, P.O. Brown, G.S. Barsh, D. Langin, In vivo regulation of human skeletal muscle gene expression by thyroid hormone. Genome Res. 12, 281–291 (2002)
W.E. Visser, K.A. Heemstra, S.M. Swagemakers, Z. Ozgür, E.P. Corssmit, J. Burggraaf, W.F. van Ijcken, P.J. van der Spek, J.W. Smit, T.J. Visser, Physiological thyroid hormone levels regulate numerous skeletal muscle transcripts. J. Clin. Endocrinol. Metab. 94, 3487–3496 (2009)
P. De Lange, R. Senese, F. Cioffi, M. Moreno, A. Lombardi, E. Silvestri, F. Goglia, A. Lanni, Rapid activation by 3,5,3′-L-triiodothyronine of adenosine 5′-monophosphate-activated protein kinase/acetyl-coenzyme A carboxylase and Akt/protein kinase B signaling pathways: relation to changes in fuel metabolism and myosin heavy-chain protein content in rat gastrocnemius muscle in vivo. Endocrinology 149, 6462–6470 (2008)
G.I. Bell, C.F. Burant, J. Takeda, G.W. Gould, Structure and function of mammalian facilitative sugar transporters. J. Biol. Chem. 268, 19161–19164 (1993)
Simpson, I.A., Dwyer, D., Malide, D., Moley, K.H., Travis, A., Vannucci, S.J.: (2008) The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. 295, E242–E253 (2008)
B.L. Ebert, J.D. Firth, P.J. Ratcliffe, Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J. Biol. Chem. 270, 29083–29089 (1995)
D.H. Lee, M.Y. Chung, J.U. Lee, D.G. Kang, J.W. Paek, Changes of glucose transporters in the cerebral adaptation to hypoglycaemia. Diabetes Res. Clin. Pract. 47, 15–23 (2000)
P. Cidad, P. Garcia-Nogales, A. Almeida, J.P. Bolanos, Expression of glucose transporter GLUT3 by endotoxin in cultured rat astrocytes: the role of nitric oxide. J. Neurochem. 79, 17–24 (2001)
M.H. Maurer, H.K. Geomor, H.F. Bürgers, D.W. Schelshorn, W. Kuschinsky, Adult neural stem cells express glucose transporters GLUT1 and GLUT3 and regulate GLUT3 expression. FEBS Lett. 580, 4430–4434 (2006)
Z.A. Khayat, A. McCall, A. Klip, Unique mechanism of GLUT3 glucose transporter regulation by prolonged energy demand: increased protein half-life. Biochem. J. 333, 713–718 (1998)
Xiao, H., Massaro, D., DeCarlo Massaro, G., Biadasz Clerch, L.: Expression of lung uncoupling protein-2 mRNA is modulated developmentally and by caloric intake. Exp. Biol. Med. 229, 479–485 (2004)
C. Cohade, M. Osman, H.K. Pannu, R.L. Wahl, Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J. Nucl. Med. 44, 170–176 (2003)
J.E. Silva, Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 86, 435–464 (2006)
J.R. Clarke, S. Brglevska, E.W. Lau, S. Ramdave, R.J. Hicks, A typical brown fat distribution in young males demonstrated on PET/CT. Clin. Nucl. Med. 32, 679–682 (2007)
A.M. Cypess, S. Lehman, G. Williams, I. Tal, D. Rodman, A.B. Goldfine, F.C. Kuo, E.L. Palmer, Y.H. Tseng, A. Doria, G.M. Kolodny, C.R. Kahn, Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009)
W.D. van Marken Lichtenbelt, J.W. Vanhommerig, N.M. Smulders, J.M. Drossaerts, G.J. Kemerink, N.D. Bouvy, P. Schrauwen, G.J.J. Teule, Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009)
K.A. Virtanen, M.E. Lidell, J. Orava, M. Heglind, R. Westergren, T. Niemi, M. Taittonen, J. Laine, N.J. Savisto, S. Enerbäck, P. Nuutila, Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009)
W.E. Visser, E.C.H. Friesema, T.J. Visser, Transport of thyroxine and 3,3′,5-triiodothyronine in human umbilical vein endothelial cells. Endocrinology 150, 1552–1557 (2009)
M.C. Skarulis, F.S. Celi, E. Mueller, M. Zemskova, R. Malek, L. Hugendubler, C. Cochran, J. Solomon, C. Chen, P. Gorden, Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J. Clin. Endocrinol. Metab. 95, 256–262 (2010)
C. Schmid, T. Steiner, E.R. Froesch, Triiodothyronine increases responsiveness of cultured rat bone cells to parathyroid hormone. Acta Endocrinol. 111, 213–216 (1986)
Acknowledgments
We thank Martina Gosteli for advice with the preparation of the GLUT cDNA probes, Oliver Tschopp for help with the statistical analysis, and Michèle Rothfuchs for help with the preparation of the manuscript. This work has been supported by the Swiss National Science Foundation (grant 32-46808.96).
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zoidis, E., Ghirlanda-Keller, C. & Schmid, C. Triiodothyronine stimulates glucose transport in bone cells. Endocrine 41, 501–511 (2012). https://doi.org/10.1007/s12020-012-9594-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9594-2