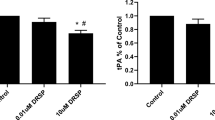
Abstract
The human placenta produces high amounts of estradiol. 17β-hydroxysteroid dehydrogenase type 2 (17βHSD2) is expressed by placental endothelial cells and was proposed to regulate sex hormone levels. Previous results obtained in term placenta suggested that 17βHSD2 expression and activity differ among umbilical cord vessels. In this study, 17βHSD2 expression level and enzymatic activity, and estrogen receptor α and β expression levels, were measured in endothelial cell cultures from umbilical arteries (HUAEC) and vein (HUVEC) using real-time quantitative PCR, western blot, and radiolabeled steroids. 17βHSD2-specific activities were also measured in proximal and distal segments of freshly isolated umbilical cord arteries and vein. 17βHSD2 mRNA level and activity were higher in HUAEC than in HUVEC. Activity was higher in umbilical arteries than in the umbilical vein. In arteries, enzymatic activity was higher near the placenta, suggesting a gradient of expression. No difference was found in ERα expression, whereas ERβ was expressed at a higher level in HUAEC than in HUVEC. Expression profiles of estrogen receptors and 17βHSD2 suggest a vessel type-specific response to estrogens. Our data support a differential modulation of biologically active sex steroid levels according to the vessel type in the foeto-placental unit, with apparent higher inactivation in the arterial system.




Similar content being viewed by others
References
E.D. Albrecht, G.J. Pepe, Placental steroid hormone biosynthesis in primate pregnancy. Endocr. Rev. 11(1), 124–150 (1990)
G.J. Pepe, E.D. Albrecht, Regulation of the primate fetal adrenal cortex. Endocr. Rev. 11(1), 151–176 (1990)
Y. Tremblay, G.E. Ringler, Y. Morel, T.K. Mohandas, F. Labrie, J.F. Strauss III, W.L. Miller, Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen—q25. J. Biol. Chem. 264(34), 20458–20462 (1989)
C. Beaudoin, C.H. Blomquist, Y. Tremblay, Gene expression of 17 beta-hydroxysteroid dehydrogenase type 2 isozyme in primary cultures of human trophoblasts predicts different mechanisms regulating type 1 and type 2 enzymes. Endocrinology 136(9), 3807–3814 (1995)
L. Wu, M. Einstein, W.M. Geissler, H.K. Chan, K.O. Elliston, S. Andersson, Expression cloning and characterization of human 17 beta-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20 alpha-hydroxysteroid dehydrogenase activity. J. Biol. Chem. 268(17), 12964–12969 (1993)
M.L. Casey, P.C. MacDonald, S. Andersson, 17 beta-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J. Clin. Invest. 94(5), 2135–2141 (1994)
M. Bonenfant, P.R. Provost, R. Drolet, Y. Tremblay, Localization of type 1 17beta-hydroxysteroid dehydrogenase mRNA and protein in syncytiotrophoblasts and invasive cytotrophoblasts in the human term villi. J. Endocrinol. 165(2), 217–222 (2000)
J. Takeyama, T. Suzuki, G. Hirasawa, Y. Muramatsu, H. Nagura, K. Iinuma, J. Nakamura, K.I. Kimura, M. Yoshihama, N. Harada, S. Andersson, H. Sasano, 17beta-hydroxysteroid dehydrogenase type 1 and 2 expression in the human fetus. J. Clin. Endocrinol. Metab. 85(1), 410–416 (2000)
M. Bonenfant, C.H. Blomquist, P.R. Provost, R. Drolet, P. D’Ascoli, Y. Tremblay, Tissue- and site-specific gene expression of type 2 17beta-hydroxysteroid dehydrogenase: in situ hybridization and specificenzymatic activity studies in human placental endothelial cells of the arterial system. J. Clin. Endocrinol. Metab. 85(12), 4841–4850 (2000)
R. Drolet, M. Simard, J. Plante, P. Laberge, Y. Tremblay, Human type 2 17 beta-hydroxysteroid dehydrogenase mRNA and protein distribution in placental villi at mid and term pregnancy. Reprod. Biol. Endocrinol. 5, 30 (2007)
J.D. Boyd, W.J. Hamilton, Development and structure of the human placenta from the end of the 3rd month of gestation. J. Obstet. Gynaecol. Br. Commonw. 74(2), 161–226 (1967)
M.E. Mendelsohn, R.H. Karas, The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 340(23), 1801–1811 (1999)
H.P. Kim, J.Y. Lee, J.K. Jeong, S.W. Bae, H.K. Lee, I. Jo, Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor alpha localized in caveolae. Biochem. Biophys. Res. Commun. 263(1), 257–262 (1999)
K.H. Kim, K. Moriarty, J.R. Bender, Vascular cell signaling by membrane estrogen receptors. Steroids 73(9–10), 864–869 (2008)
T. Hayashi, K. Yamada, T. Esaki, M. Kuzuya, S. Satake, T. Ishikawa, H. Hidaka, A. Iguchi, Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem. Biophys. Res. Commun. 214(3), 847–855 (1995)
M. Akishita, K. Kozaki, M. Eto, M. Yoshizumi, M. Ishikawa, K. Toba, H. Orimo, Y. Ouchi, Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem. Biophys. Res. Commun. 251(1), 17–21 (1998)
M.A. Herve, G. Meduri, F.G. Petit, T.S. Domet, G. Lazennec, S. Mourah, M. Perrot-Applanat, Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J. Endocrinol. 188(1), 91–99 (2006)
B. Toth, G. Saadat, A. Geller, C. Scholz, S. Schulze, K. Friese, U. Jeschke, Human umbilical vascular endothelial cells express estrogen receptor beta (ERbeta) and progesterone receptor A (PR-A), but not ERalpha and PR-B. Histochem. Cell Biol. 130(2), 399–405 (2008)
A.H. Wagner, M.R. Schroeter, M. Hecker, 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 15(12), 2121–2130 (2001)
A. Sobrino, M. Mata, A. Laguna-Fernandez, S. Novella, P.J. Oviedo, M.A. Garcia-Perez, J.J. Tarin, A. Cano, C. Hermenegildo, Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells. PLoS One 4(12), e8242 (2009)
W. Tschugguel, W. Dietrich, Z. Zhegu, F. Stonek, A. Kolbus, J.C. Huber, Differential regulation of proteasome-dependent estrogen receptor alpha and beta turnover in cultured human uterine artery endothelial cells. J. Clin. Endocrinol. Metab. 88(5), 2281–2287 (2003)
E.A. Jaffe, R.L. Nachman, C.G. Becker, C.R. Minick, Culture of human endothelial cells derived from umbilical veins Identification by morphologic and immunologic criteria. J. Clin. Invest. 52(11), 2745–2756 (1973)
R.T. Hinegardner, An improved fluorometric assay for DNA. Anal. Biochem. 39(1), 197–201 (1971)
B. Fiszer-Szafarz, D. Szafarz, A. Guevara de Murillo, A general, fast, and sensitive micromethod for DNA determination application to rat and mouse liver, rat hepatoma, human leukocytes, chicken fibroblasts, and yeast cells. Anal. Biochem. 110(1), 165–170 (1981)
C.H. Blomquist, H.C. Hensleigh, D.L. Block, L.A. Feeney, Placental 17 beta-hydroxysteroid oxidoreductase, lactate dehydrogenase and malate dehydrogenase during the latter half of pregnancy in the mouse. J. Steroid Biochem. Mol. Biol. 46(1), 61–67 (1993)
M. Simard, J. Plante, M. Boucher, P.R. Provost, Y. Tremblay, Type 2 and 5 17beta-hydroxysteroid dehydrogenases and androgen receptor in human fetal lungs. Mol. Cell. Endocrinol. 319(1–2), 79–87 (2010)
M. Simard, E. Boucher, P.R. Provost, Y. Tremblay, Minimization of PCR efficiency differences between standards and samples through dilution of PCR amplicons in reverse transcription buffer. Anal. Biochem. 362(1), 142–144 (2007)
J. Vandesompele, K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, F. Speleman, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3(7), RESEARCH0034 (2002)
C.H. Blomquist, D.G. Bealka, H.C. Hensleigh, G.E. Tagatz, A comparison of 17 beta-hydroxysteroid oxidoreductase type 1 and type 2 activity of cytosol and microsomes from human term placenta, ovarian stroma and granulosa-luteal cells. J. Steroid Biochem. Mol. Biol. 49(2–3), 183–189 (1994)
S.S. Khosla, S.A. Rooney, Stimulation of fetal lung surfactant production by administration of 17beta-estradiol to the maternal rabbit. Am. J. Obstet. Gynecol. 133(2), 213–216 (1979)
S.S. Khosla, G.J. Smith, P.A. Parks, S.A. Rooney, Effects of estrogen on fetal rabbit lung maturation: morphological and biochemical studies. Pediatr. Res. 15(9), 1274–1281 (1981)
K. Hanley, U. Rassner, Y. Jiang, D. Vansomphone, D. Crumrine, L. Komuves, P.M. Elias, K.R. Feingold, M.L. Williams, Hormonal basis for the gender difference in epidermal barrier formation in the fetal rat. Acceleration by estrogen and delay by testosterone. J. Clin. Invest. 97(11), 2576–2584 (1996)
M.L. Williams, K. Hanley, P.M. Elias, K.R. Feingold, Ontogeny of the epidermal permeability barrier. J. Investig. Dermatol. Symp. Proc. 3(2), 75–79 (1998)
J.A. Visser, A. McLuskey, M. Verhoef-Post, P. Kramer, J.A. Grootegoed, A.P. Themmen, Effect of prenatal exposure to diethylstilbestrol on Mullerian duct development in fetal male mice. Endocrinology 139(10), 4244–4251 (1998)
J.R. Palmer, E.E. Hatch, R.S. Rao, R.H. Kaufman, A.L. Herbst, K.L. Noller, L. Titus-Ernstoff, R.N. Hoover, Infertility among women exposed prenatally to diethylstilbestrol. Am. J. Epidemiol. 154(4), 316–321 (2001)
H.W. Horne Jr., Evidence of improved pregnancy outcome with diethylstilbestrol (DES) treatment of women with previous pregnancy failures: a retrospective analysis. J. Chronic Dis. 38(10), 873–880 (1985)
H.W. Horne Jr., R.B. Kundsin, Results of infertility studies on DES-exposed and non DES-exposed consecutive patients. Int. J. Fertil. 30(1), 46–49 (1001). (1985)
R.M. Giusti, K. Iwamoto, E.E. Hatch, Diethylstilbestrol revisited: a review of the long-term health effects. Ann. Intern. Med. 122(10), 778–788 (1995)
G. Delbes, C. Levacher, R. Habert, Estrogen effects on fetal and neonatal testicular development. Reproduction 132(4), 527–538 (2006)
L.J. Guillette Jr., B.C. Moore, Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin. Reprod. Med. 24(3), 134–141 (2006)
M. Mustonen, M. Poutanen, A. Chotteau-Lelievre, Y. de Launoit, V. Isomaa, S. Vainio, R. Vihko, P. Vihko, Ontogeny of 17beta-hydroxysteroid dehydrogenase type 2 mRNA expression in the developing mouse placenta and fetus. Mol. Cell. Endocrinol. 134(1), 33–40 (1997)
M. Mustonen, V.V.T. Isomaa, J. Tapanainen, M. Poutanen, F. Stenbäck, R. Vihko, P. Vihko, Human 17beta-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 83(4), 1319–1324 (1998)
A. Bukovsky, M.R. Caudle, M. Cekanova, R.I. Fernando, J. Wimalasena, J.S. Foster, D.C. Henley, R.F. Elder, Placental expression of estrogen receptor beta and its hormone binding variant—comparison with estrogen receptor alpha and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod. Biol. Endocrinol. 1, 36 (2003)
B. Schiessl, I. Mylonas, C. Kuhn, S. Kunze, S. Schulze, K. Friese, U. Jeschke, Expression of estrogen receptor-alpha, estrogen receptor-beta and placental endothelial and inducible NO synthase in intrauterine growth-restricted and normal placentals. Arch. Med. Res. 37(8), 967–975 (2006)
Acknowledgments
We thank the nurses from the Centre Mère-Enfant at the Centre Hospitalier Universitaire de Québec for their precious collaboration. We also thank Dr. Martin Bonenfant, PhD, for technical support, Julie Plante, MSc, for technical support and for critical reading of the manuscript, and Dr. Richard Poulin, PhD, for editing of the manuscript. This study was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC 171140-05) and the Canadian Institutes of Health Research (MOP 84221) to YT. This manuscript is dedicated to the memory of Dr Charles H Blomquist, deceased in September 2009.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simard, M., Drolet, R., Blomquist, C.H. et al. Human type 2 17beta-hydroxysteroid dehydrogenase in umbilical vein and artery endothelial cells: differential inactivation of sex steroids according to the vessel type. Endocrine 40, 203–211 (2011). https://doi.org/10.1007/s12020-011-9519-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-011-9519-5