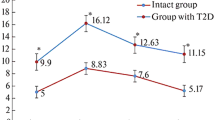
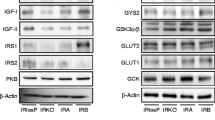
Abstract
Pancreatic β cells, stimulated by glucose, are known to synthesize and secrete insulin. As liver diseases are reported to cause diabetes mellitus, studies were conducted to determine the possibility of glucose-induced insulin synthesis in the liver cells. The glucose-induced insulin synthesis was determined by in vitro translation of mRNA from the hepatocytes. The cDNA from mRNA was prepared and sequence analysis was performed. Incubation of hepatocytes from the liver of adult mice (n = 10) with glucose (0.02 M) resulted in the insulin synthesis [0.03 (mean) ± 0.006 (S.D.) μunits/mg/h] compared to the pancreatic β cells [0.04 ± 0.004 μunits/mg/h]. Immunohistochemical study also demonstrated the glucose-induced synthesis of insulin in liver cells. Incubation of the mice hepatocytes with glucose resulted in the synthesis of insulin mRNA. The purified mRNA which was used to prepare cDNA resulted in the formation of proinsulin I and proinsulin II genes corresponding to 182 and 188 base pairs, respectively. Sequence analysis of the cDNA indicated that proinsulin I as well as proinsulin II gene could be involved in the synthesis of insulin by hepatocytes. These results suggested that insulin synthesis in both hepatic and pancreatic cells could be involved in the control of diabetes mellitus.







Similar content being viewed by others
References
G.M. Grodsky, A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J. Clin. Invest. 51(8), 2047–2059 (1972)
K. Asplund, S. Westman, C. Hellerström, Glucose stimulation of insulin secretion from the isolated pancreas of foetal and newborn rats. Diabetologia 5(4), 260–262 (1969)
P. Louise Deltour, N. Leduque, O. Blume, P. Madsen, J. Duboi, Jamiand D. Bucchini, Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo (PCR/immunocytochemistry). Proc. Natl. Acad. Sci. USA 90, 527–531 (1993)
D. Bruemmer, C-Peptide in insulin resistance and vascular complications: teaching an old dog new tricks. Circ. Res. 99(11), 1149–1151 (2006)
C. Meyer, J.M. Dostou, S.L. Welle, J.E. Gerich, Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 282, E419–E427 (2002)
G.N. Levinthal, A.S. Tavill, Liver disease and diabetes mellitus. Diabetes 17, 2 (1999)
J.M. Petit, J.B. Bour, C. Galland-Jos, A. Minello, B. Verges, M. Guiguet, J.M. Brun, P. Hillon, Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J. Hepatol. 35(2), 279–283 (2001)
L. Yang, S. Li, H. Hatch, K. Ahrens, J.G. Cornelius, B.E. Petersen, A.B. Peck, In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. USA 99(12), 8078–8083 (2002)
J.L. Rosenzweig, J. Havrankova, M.A. Lesniak, M. Brownstein, J. Roth, Insulin is ubiquitous in extrapancreatic tissues of rats and humans. Proc. Natl. Acad. Sci. USA 77(1), 572–576 (1980)
A. Blouin, R.P. Bolender, E.R. Weibel, Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. J. Cell Biol. 72, 441–455 (1977)
M.T. Guillam, R. Burcelin, B. Thorens, Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc. Natl. Acad. Sci. USA 95(21), 12317–12321 (1998)
E.R. Arquilla, A.B. Stavitsky, The production and identification of antibodies to insulin and their use in assaying insulin. J. Clin. Investig. 35(5), 458–466 (1956)
P.E. Lacy, M. Kostianovsky, Method for isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16, 35–39 (1967)
Evan Breda, H.A. Keizer, J.F. Glatz, P. Geurten, Use of the intact mouse skeletal-muscle preparation for metabolic studies. Evaluation of the model. Biochem. J. 267(1), 257–260 (1990)
A. Virkamäki, K. Ueki, C.R. Kahn, Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Invest. 103(7), 931–943 (1999)
J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, W. Strober (eds.), Current Protocolin Immunology, vol. 1, section 5.8 (Wiley, New York, 1992). (supported by NIH)
E. Engvall, P. Perlmann, Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 109(1), 129–135 (1972)
H.J. Baelde, A.M. Cleton-Jansen, H. van Beerendonk, M. Namba, J.V. Bovée, P.C. Hogendoorn, High quality RNA isolation from tumors with low cellularity and high extracellular matrix component for cDNA microarrays: application to chondrosarcoma. J. Clin. Pathol. 54, 778–782 (2001)
R. Zimmerman, U. Paluch, M. Sprinzl, W. Neupert, Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur. J. Biochem. 99, 247–252 (1979)
R. Zhang, J. Zhou, Z. Jia, Y. Zhang, G. Gu, Hypoglycemic effect of Rehmannia glutinosa oligosaccharide in hyperglycemic and alloxan-induced diabetic rats and its mechanism. J. Ethnopharmacol. 90, 39–43 (2004)
E.M. Scolnick, R. Benveniste, W.P. Parks, Purification by oligo(dT)-cellulose of viral-specific RNA from sarcoma virus-transformed mammalian nonproducer cells. J. Virol. 11(4), 600–602 (1973)
B. Cordell, G. Bell, E. Tischer, F.M. DeNoto, A. Ullrich, R. Pictet, W.J. Rutter, H.M. Goodman, Isolation and characterization of a cloned rat insulin gene. Cell 18(2), 533–543 (1979)
P. Borst, Ethidium DNA agarose gel electrophoresis: how it started. IUBMB Life 57(11), 745−747 (2005)
S.F. Altschul, W. Gish, W. Miller, E.W. Myers, D.J. Lipman, Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990)
O. Hrytsenko, J.R. Wright Jr, C.M. Morrison, B. Pohajdak, Insulin expression in the brain and pituitary cells of tilapia (Oreochromis niloticus). Brain Res. 1135(1), 31–40 (2007)
A.C. Powers, (2005) in Diabetes Mellitus, vol. II, ed. by D.L. Kasper, A.S. Fauci, D.L. Longo, E. Braunwald, S.L. Hauser, J.L. Jameson. Harrison’s Principles of Internal Medicine vol. II, 16th edn. pp. 2155
I.A. Mirsky, G. Perisutti, The relative specificity of the insulinase activity of rat liver extracts. J. Biol. Chem. 228(1), 77–83 (1957)
M. Fehlmann, J.L. Carpentier, E. Van Obberghen, P. Freychet, P. Thamm, D. Saunders, D. Brandenburg, L. Orci, Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc. Natl. Acad. Sci. USA 79(19), 5921–5925 (1982)
L. Jarett, J.B. Schweitzer, R.M. Smith, Insulin receptors: differences in structural organization on adipocyte and liver plasma membranes. Science 210, 1127–1128 (1980)
F.D. Davison, D.W.R. Mackenzie, DNA homology studies in the taxonomy of dermatophytes. Med. Mycol. 22, 117–123 (1984)
J. Zhou, M.X. Shi, T.D. Mitchell, G.N. Smagin, S.R. Thomas, D.H. Ryan, R.B.S. Harris, Changes in rat adipocyte and liver glucose metabolism following repeated restraint stress. Exp. Biol. Med. 226, 312–319 (2001)
P. Matsudaira, Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262, 10035–10038 (1987)
U.K. Laemmli, Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 27, 680–685 (1970)
O. Orjan, 2007 Antigen-antibody reactions in gels. APMIS 115(5), 486–495, 496–498 (1952)
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this work was published as an abstract in the proceeding of the Annual Workshop in Biological Sciences, Calcutta, 2009.
An erratum to this article can be found at http://dx.doi.org/10.1007/s12020-011-9571-1
Rights and permissions
About this article
Cite this article
Ghosh, R., Karmohapatra, S.K., Bhattacharya, G. et al. The glucose-induced synthesis of insulin in liver. Endocr 38, 294–302 (2010). https://doi.org/10.1007/s12020-010-9388-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-010-9388-3