Abstract
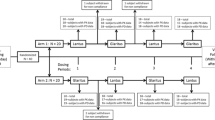
Intensive insulin therapy for diabetic patients has been demonstrated as an appropriate treatment. Regular fast-acting insulin can hardly mimic the efficiency of endogenous meal-activated insulin secretion. Glulisine is a new rapid-acting insulin analog for mealtime insulin supplementation. We compared the pharmacokinetics and pharmacodynamics end points between the two rapid-acting insulin analogs Glulisine and Lispro. Twenty healthy adult males age ranging from 22 to 32 years were included in a randomized, open-label, cross contrast research. Two long duration hyperinsulinemic euglycemic clamp tests, one with Glulisine and the other with Lispro, were conducted on two separate days for all the participants. The two rapid-acting insulin analogs were administrated randomly to each participant. Glucose infusion rate (GIR) began to increase 20 min after injection in both Glulisine and Lispro groups. GIR increased sharply during the first 150 min and reached a peak at 6.23 ± 1.35 mg/(kg min) in the Glulisine group and 6.02 ± 1.27 mg/(kg min) in the Lispro group. It returned to the initial level at hour 5. The Area Under Curve (AUC0-clamp end) in Glulisine and Lispro groups were 1455.04 ± 381.88 mg/kg and 1356.25 ± 287.30 mg/kg (P > 0.05), respectively. However, AUC0–1h between the two groups showed significant difference, with Glulisine showed greater AUC0–1h in the first hour after injection. Other parameters showed no significant difference between the two groups. Insulin analogs Glulisine and Lispro were proved to have equivalent pharmacokinetic and pharmacodynamic parameters when administered to healthy Chinese adults, but with Glulisine showing greater AUC0–1h after injection.


Similar content being viewed by others
References
P. Reichard, B.Y. Nilsson, U. Rosenquist, The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N. Engl. J. Med. 329, 304–309 (1993)
The Diabetes Control and Complications Trial Research Group, The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993)
S. Pampanelli, C. Fanelli, C. Lalli et al., Long-term intensive insulin therapy: effects of HbA1c, risk for severe and mild hypoglycemia, status of counterregulation and unawareness of hypoglycemia. Diabetologia 39, 677–686 (1996)
S. Bott, U. Bott, M. Berger, I. Mülhauser, Intensified insulin therapy and the risk of severe hypoglycaemia. Diabetologia 40, 926–932 (1997)
N.P. Murphy, J.A. Edge, S.M. Keane et al., Randomized cross-over trial of insulin Glargine plus Lispro or NPH insulin plus regular human insulin in adolescents with Type 1 diabetes on intensive insulin regimens. Diab. Care 26, 799–804 (2003)
G.B. Bolli, R. Di Marchi, G. Park, S. Pramming, V.A. Koivisto, Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia 42, 1151–1167 (1999)
A.M. Hennige, R. Lehmann, C. Weigert et al., Insulin receptor signaling characteristics in vivo. Diabetes 54, 361–366 (2005)
Noble SL, Johnston E, Walton B (1998) Insulin Lispro: a fast-acting insulin analog. Am Fam Physician. 57, 279–286, 289–292
P.M. Jehle, R.D. Fussgaenger, U. Kunze et al., The human insulin analog insulin lispro improves insulin binding on circulating monocytes of intensively treated insulin-dependent diabetes mellitus patients. J. Clin. Endocrinol. Metab. 81, 2319–2327 (1996)
M. Lepore, S. Pampanelli, C. Fanelli et al., Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 49, 2142–2148 (2000)
Insulin Aspart, a new rapid-acting insulin. Med. Lett Drugs Ther. 43, 89–90 (2001)
D.C. Howey, R.R. Bowsher, R.L. Brunelle et al., [Lys(B28), Pro(B29)] human insulin: a rapidly absorbed analogue of human insulin. Diabetes 43, 396–402 (1994)
R.H. Becker, A.D. Frick, Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin. Pharmacokinet. 47, 7–20 (2008)
R.H. Becker, A.D. Frick, F. Burger et al., Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp. Clin. Endocrinol. Diab. 113, 435–443 (2005)
T. Heise, L. Nosek, H. Spitzer et al., Insulin glulisine: a faster onset of action compared with insulin lispro. Diab. Obes. Metab. 9, 746–753 (2007)
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 30700383, No. 30725037) and the Shanghai Education Commission (No. Y0204, No. E03007). We are grateful to Aventis Pharma Deutschland GmbH and Lily France S.A.S for providing the experimental products Glulisne and Lispro, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Menglei Chao and Weiqing Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chao, M., Wang, W., Zhang, Y. et al. Bioequivalence between two human insulin analogs in Chinese population: Glulisine and Lispro. Endocr 38, 48–52 (2010). https://doi.org/10.1007/s12020-010-9326-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-010-9326-4