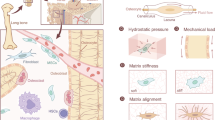
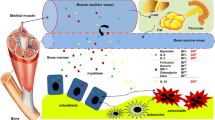
Abstract
The complexity of cell interactions with their microenvironment and their ability to communicate at the autocrine, paracrine, and endocrine levels has gradually but significantly evolved in the last three decades. The musculoskeletal system has been historically recognized to be governed by a relationship of proximity and function, chiefly dictated by mechanical forces and the work of gravity itself. In this review article, we first provide a historical overview of the biomechanical theory of bone–muscle interactions. Next, we expand to detail the significant evolution in our understanding of the function of bones and muscles as secretory organs. Then, we review and discuss new evidence in support of a biochemical interaction between these two tissues. We then propose that these two models of interaction are complementary and intertwined providing for a new frontier for the investigation of how bone–muscle cross talk could be fully explored for the targeting of new therapies for musculoskeletal diseases, particularly the twin conditions of aging, osteoporosis and sarcopenia. In the last section, we explore the bone–muscle cross talk in the context of their interactions with other tissues and the global impact of these multi-tissue interactions on chronic diseases.


Similar content being viewed by others
References
Ioannidis I. The road from obesity to type 2 diabetes. Angiology. 2008;59(Supp 2):39S–43S.
Berg AH, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–53.
Sell H, Dietze-Schroeder D, Eckel J. The adipocyte–myocyte axis in insulin resistance. Trends Endocrinol Metab. 2006;17(10):416–22.
Lafontan M. Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. AJP Cell Physiol. 2011;302:C327–59.
Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9:584–97.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years live with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96.
Frost HM. Perspectives: a proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm). Anat Rec. 1996;244:139–47.
Marotti G, Ferretti M, Muglia MA, Palumbo C, Palazzini S. A quantitative evaluation of osteoblast–osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone. 1992;13:363–8.
Tanaka K, Matsuo T, Ohta M, Sato T, Tezuka K, Nijweide PJ, et al. Time-lapse microcinematography of osteocytes. Miner Electrolyte Metab. 1995;21:189–92.
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASB J. 1995;9:441–5.
Burger EH, Klein-Nulend J. Mechanotransduction in bone—role of the lacuna-canalicular network. FASEB J. 1999;13:101–12.
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of one formatin via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–76.
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiologyical role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8.
Bonewald LF, Wacker MJ. FGF23 production by osteocytes. Pediatr Nephrol. 2012;28:563–8.
Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–62.
Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406.
Lajeunesse D, Kiebzak GM, Frondoza C, Sacktor B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Miner. 1991;14:237–50.
Ducy P, Desbois C, Boyce B, Pinero G, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69.
Agas D, Marchetti L, Hurley MM, Sabbieti MG. Prostablandin F2α: a bone remodeling mediator. J Cell Physiol. 2013;228:25–9.
Mo C, Romero-Suarez S, Brotto MA. Pge2 accelerates myogenesis of C2C12 myoblasts. Biophys J. 2011;100:288a.
Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20.
Orestes-Cardoso SM, Nefussi JR, Hotton D, Mesbah M, Orestes-Cardoso MDS, Robert B, et al. Postnatal Msx1 expression pattern in craniofacial, axial, and appendicular skeleton of transgenic mice from the first week until the second year. Dev Dyn. 2001;221:1–13.
Pearson OM, Lieberman DE. The aging of Wolff’s law: ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004;125:63–99.
Lang TF. The bone–muscle relationship in men and women. J Osteoporos. 2011;2011:1–4.
Zanchetta JR, Plotkin H, Filgueira MLA. Bone mass in children: normative values for the 2–20-year-old population. Bone. 1995;16:S393–9.
Wang Q, Alen M, Nicholson P, Suominen H, Koistinen A, Kroger H, et al. Weight-bearing, muscle loading and bone mineral accrual in pubertal girls—a 2-year longitudinal study. Bone. 2007;40:1196–202.
Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–38.
Gomez-Pinilla F. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–95.
Goldspink DF, Goldspink G. The role of passive stretch in retarding muscle atrophy. In: Nix WA, Vrbova G, editors. Electrical stimulation and neuromuscular disorders. Berlin: Springer; 1986. p. 91–100.
Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, Austin L. The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve. 1997;20:815–22.
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24:113–9.
Steensberg A, Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–42.
Andersen K, Pedersen B. The role of inflammation in vascular insulin resistance with focus on IL-6. Horm Metab Res. 2008;40:635–9.
Keller C. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15(14):2748–50.
Nielsen AR, Pedersen BK. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007;32:833–9.
Pedersen BK, Akerstrom TCA, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8.
Matthews VB, Astrom MB, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18.
Pedersen L, Olsen CH, Pedersen BK, Hojman P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. AJP Endocrinol Metab. 2012;302:E831–40.
Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7.
Hee Park K, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(12):4899–907.
Chan JKK, Harry L, Williams G, Nanchahal J. Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plast Reconstr Surg. 2012;130:284e–95e.
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature. 1997;387:83–90.
Jouliaekaza D, Cabello G. The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol. 2007;7:310–5.
Zimmers TA. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–8.
Hamrick MW, McPherron AC, Lovejoy CO. Bone mineral content and density in humerus of adult myostatin-deficient mice. Calcif Tissue Int. 2002;71:63–8.
Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec. 2003;272A:388–91.
Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2005;21:477–83.
Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, et al. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8:573–83.
Coiro V, Volpi R, Cataldo S, Magotti MG, Maffei ML, Giumelli C, et al. Effect of physiological exercise on osteocalcin levels in subjects with adrenal incidentaloma. J Endocrinol Invest. 2012;35:357–8.
Centers for Disease Control and Prevention (US); Prevalence of obesity among older adults in the United States, 2007–2010. 2012. (NCHS Data Brief; no. 106).
Beltran-Sanchez H, Harhay MO, Harhay MM, McElliogott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703.
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–43.
Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–48.
Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96(5):895–901.
Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4:89–94.
Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48.
Jensen GL, Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50(5):918–23.
Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5(5):1–7.
Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes. Diabetes Care. 2010;33(7):1497–9.
Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26(7–8):686–93.
Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59:1217–24.
Hofbauer LG, Brueck C, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. JBMR. 2007;22(9):1317–28.
Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14:429–35.
Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84:45–55.
Hamann C, Kirschner S, Gunther KP, Hofbauer LC. Bone sweet bone—osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297–305.
Acknowledgments
This work was supported by the NIH-National Institutes of Aging Program Project Grant P01 AG039355-01-A1, Missouri Life Sciences Research Board and the Thompson Endowment Fund (MB). We are thankful to the members of the UMKC Bone Biology Group and Muscle Biology Group for useful discussions that were helpful in the preparation of this manuscript.
Disclosures
Conflict of interest
Janalee Isaacson and Marco Brotto declare that they have no conflict of interest.
Animal/Human Studies
This review does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isaacson, J., Brotto, M. Physiology of Mechanotransduction: How Do Muscle and Bone “Talk” to One Another?. Clinic Rev Bone Miner Metab 12, 77–85 (2014). https://doi.org/10.1007/s12018-013-9152-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-013-9152-3