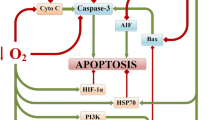
Abstract
The mechanisms and signaling pathways of the neuroprotective effects of hypercapnia and its combination with hypoxia are not studied sufficiently. The study aims to test the hypothesis of the potentiating effect of hypercapnia on the systems of adaptation to hypoxia, directly associated with A1-adenosine receptors and mitochondrial ATP-dependent K+ -channels (mitoK+ATP-channels). We evaluated the relative number of A1-adenosine receptors and mitoK+ATP-channels in astrocytes obtained from male Wistar rats exposed to various respiratory conditions (15 times of hypoxia and/or hypercapnia). In addition, the relative number of these molecules in astrocytes was evaluated on an in vitro model of chemical hypoxia, as well as in the cerebral cortex after photothrombotic damage. This study indicates an increase in the relative number of A1-adenosine receptors in astrocytes and in cells next to the stroke region of the cerebral cortex in rats exposed to hypoxia and hypercapnic hypoxia, but not hypercapnia alone. Hypercapnia and hypoxia increase the relative number of mitoK+ATP-channels in astrocytes and in cells of the peri-infarct region of the cerebral cortex in rats. In an in vitro study, hypercapnia mitigates the effects of acute chemical hypoxia observed in astrocytes for A1-adenosine receptors and mitoK+ATP-channels. Hypercapnia, unlike hypoxia, does not affect the relative number of A1 receptors to adenosine. At the same time, both hypercapnia and hypoxia increase the relative number of mitoK+ATP-channels, which can potentiate their protective effects with combined exposure.
Graphic Abstract








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Ahmet, I., Krawczyk, M., Heller, P., Moon, C., Lakatta, E. G., & Talan, M. I. (2004). Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation, 110(9), 1083–1090.
Baillieul, S., Chacaroun, S., Doutreleau, S., Detante, O., Pépin, J. L., & Verges, S. (2017). Hypoxic conditioning and the central nervous system: A new therapeutic opportunity for brain and spinal cord injuries? Experimental Biology and Medicine (Maywood, N.J.), 242(11), 1198–1206.
Barth, A. M., & Mody, I. (2011). Changes in hippocampal neuronal activity during and after unilateral selective hippocampal ischemia in vivo. Journal of Neuroscience, 31, 851–860.
Barth, A., Bauer, R., Gedrange, T., Walter, B., Klinger, W., & Zwiener, U. (1998). Influence of hypoxia and hypoxia/hypercapnia upon brain and blood peroxidative and glutathione status in normal weight and growth-restricted newborn piglets. Experimental and Toxicologic Pathology, 50(4–5), 402–410.
Bespalov, A. G., Tregub, P. P., Kulikov, V. P., Pijanzin, A. I., & Belousov, A. A. (2014). The role of VEGF, HSP-70 and protein S-100B in the potentiation effect of the neuroprotective effect of hypercapnic hypoxia. Patologicheskaia Fiziologiia i Eksperimentalnaia Terapiia, 2, 24–27.
Björklund, O., Shang, M., Tonazzini, I., Daré, E., & Fredholm, B. B. (2008). Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. European Journal of Pharmacology, 596(1–3), 6–13.
Cao, T., Ma, T., Xu, Y., Tian, Y., Cai, Q., Li, B., & Li, H. (2019). Caffeine treatment promotes differentiation and maturation of hypoxic oligodendrocytes via counterbalancing adenosine 1 adenosine receptor-induced calcium overload. Medical Science Monitor, 25, 1729–1739.
Coppi, E., Dettori, I., Cherchi, F., Bulli, I., Venturini, M., Lana, D., Giovannini, M. G., Pedata, F., & Pugliese, A. M. (2020). A2B adenosine receptors: When outsiders may become an attractive target to treat brain ischemia or demyelination. International Journal of Molecular Sciences, 21(24), 9697.
Chen, W. J., Chen, H. W., Yu, S. L., Huang, C. H., Wang, T. D., Chen, J. J., Chien, C. T., Chen, H. Y., Yang, P. C., & Lee, Y. T. (2005). Gene relative number profiles in hypoxic preconditioning using cDNA microarray analysis: Altered relative number of an angiogenic factor, carcinoembryonic antigen-related cell adhesion molecule 1. Shock, 24, 124–131.
Dahlem, Y., Horn, T., Butinas, L., Gonoi, T., Wolf, T., & Siemen, D. (2004). The human mitochondrial KATP channel is modulated by calcium and nitric oxide: A patch-clamp approach. Biochimica et Biophysica Acta, 1656, 46–56.
Dell, R. B., Holleran, S., & Ramakrishnan, R. (2002). Sample size determination. ILAR Journal, 43, 207–213.
Deryagin, O. G., Gavrilova, S. A., Gainutdinov, K. L., Golubeva, A. V., Andrianov, V. V., Yafarova, G. G., Buravkov, S. V., & Koshelev, V. B. (2017). Molecular bases of brain preconditioning. Frontiers in Neuroscience, 11, 427.
Faraco, C. C., Strother, M. K., Siero, J. C., Arteaga, D. F., Scott, A. O., Jordan, L. C., & Donahue, M. J. (2015). The cumulative influence of hyperoxia and hypercapnia on blood oxygenation and R2. Journal of Cerebral Blood Flow and Metabolism, 35(12), 2032–2042.
Garlid, K. D., & Beavis, A. D. (1986). Evidence for the existence of an inner membrane anion channel in mitochondria. Biochimica et Biophysica Acta, 273, 13578–13582.
Goss, S. P., Singh, R. J., & Kalyanaraman, B. (1999). Bicarbonate enhances the peroxidase activity of Cu, Zn-superoxide dismutase. Role of carbonate anion radical. Journal of Biological Chemistry, 274(40), 28233–28239.
Haubrich, C., Steiner, L., Kasprowicz, M., Diedler, J., Carrera, E., Diehl, R. R., Smielewski, P., & Czosnyka, M. (2011). Short-term moderate hypocapnia augments detection of optimal cerebral perfusion pressure. Journal of Neurotrauma, 28(7), 1133–1137.
Heurteaux, C., Lauritzen, I., Widmann, C., & Lazdunski, M. (1995). Essential role of adenosine, adenosine A1 receptors, ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proceedings of the National Academy of Sciences USA, 92(10), 4666–4670.
Howell, N. J., & Tennant, D. (2014). The role of HIFs in ischemia-reperfusion injury. Hypoxia, 2, 107–115.
Ilie, A., Ciocan, D., Zagrean, A. M., Nita, D. A., Zagrean, L., & Moldovan, M. (2006). Endogenous activation of adenosine A1 receptors accelerates ischemic suppression of spontaneous electrocortical activity. Journal of Neurophysiology, 96(5), 2809–2814.
Jacobson, K. A., & Gao, Z. G. (2006). Adenosine receptors as therapeutic targets. Nature Reviews Drug Discovery, 5, 247–264.
Kniffin, C. D., Burnett, L. E., & Burnett, K. G. (2014). Recovery from hypoxia and hypercapnic hypoxia: Impacts on the transcription of key antioxidants in the shrimp Litopenaeus vannamei. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 170, 43–49.
Krenz, M., Oldenburg, O., Wimpee, H., Cohen, M. V., Garlid, K. D., Critz, S. D., Downey, J. M., & Benoit, J. N. (2002). Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Research in Cardiology, 97, 365–373.
Kulikov, V. P., Tregub, P. P., Kovzelev, P. D., Dorokhov, E. A., & Belousov, A. A. (2015). Hypercapnia-alternative hypoxia signal incentives to increase HIF-1α and erythropoietin in the brain. Patologicheskaia Fiziologiia i Eksperimentalnaia Terapiia, 3, 34–37.
Kulinskiĭ, V. I., Gavrilina, T. V., Minakina, L. N., & Kovtun, VIu. (2006). Biochemical and pharmacological mechanisms of different types of hypoxic preconditioning in cerebral ischemia in mice. Biomeditsinskaya Khimiya, 52(3), 309–316.
Kuvacheva, N. V., Morgun, A. V., Komleva, Y. K., Khilazheva, E. D., Gorina, Y. V., Lopatina, O. L., Arutyunyan, S. A., & Salmina, A. B. (2015). In vitro modeling of brain progenitor cell development under the effect of environmental factors. Bulletin of Experimental Biology and Medicine, 159(4), 546–549.
Lacza, Z., Snipes, J., Kis, B., Szabo, C., Grover, G., & Busija, D. (2003). Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Research, 994, 27–36.
Laffey, J. G., Tanaka, M., Engelberts, D., Luo, X., Yuan, S., Tanswell, A. K., Post, M., Lindsay, T., & Kavanagh, B. P. (2000). Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. American Journal of Respiratory and Critical Care Medicine, 162, 2287–2294.
Leak, R. K., Calabrese, E. J., Kozumbo, W. J., Gidday, J. M., Johnson, T. E., Mitchell, J. R., Ozaki, C. K., Wetzker, R., Bast, A., Belz, R. G., Bøtker, H. E., Koch, S., Mattson, M. P., Simon, R. P., Jirtle, R. L., & Andersen, M. E. (2018). Enhancing and extending biological performance and resilience. Dose Response, 16, 1559325818784501. https://doi.org/10.1177/1559325818784501
Li, B., & Roth, S. (1999). Retinal ischemic preconditioning in the rat: Requirement for adenosine and repetitive induction. Investigative Ophthalmology & Visual Science, 40(6), 1200–1216.
Li, H. L., Zaghloul, N., Ahmed, I., Omelchenko, A., Firestein, B. L., Huang, H., & Collins, L. (2019). Caffeine inhibits hypoxia-induced nuclear accumulation in HIF-1α and promotes neonatal neuronal survival. Experimental Neurology, 317, 66–77.
Lindauer, U., Vogt, J., Schuh-Hofer, S., Dreier, J. P., & Dirnagl, U. (2003). Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. Journal of Cerebral Blood Flow and Metabolism, 23(10), 1227–1238.
Liu, Z., & Chopp, M. (2016). Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Progress in Neurobiology, 144, 103–120.
Liu, C., Cao, Y., Malhotra, A., & Ling, L. (2011). Sleep fragmentation attenuates the hypercapnic (but not hypoxic) ventilatory responses via adenosine A1 receptors in awake rats. Respiratory Physiology & Neurobiology, 175(1), 29–36.
Lukyanova, L. D., Germanova, E. L., & Kopaladze, R. A. (2009). Development of resistance of an organism under various conditions of hypoxic preconditioning: Role of the hypoxic period and reoxygenation. Bulletin of Experimental Biology and Medicine, 147(4), 400–404.
Martí Navia, A., Dal Ben, D., Lambertucci, C., Spinaci, A., Volpini, R., Marques-Morgado, I., Coelho, J. E., Lopes, L. V., Marucci, G., & Buccioni, M. (2020). Adenosine receptors as neuroinflammation modulators: Role of A1 agonists and A2A antagonists. Cells, 9(7), 1739.
Malyshev, I. Y., Zenina, T. A., Golubeva, L. Y., Saltykova, V. A., Manukhina, E. B., Mikoyan, V. D., Kubrina, L. N., & Vanin, A. F. (1999). NO-dependent mechanisms of adaptation to hypoxia. Nitric Oxide, 3, 105–113.
Maslov, L. N., Lishmanov, Y. B., Oeltgen, P. R., Barzakh, E. I., Krylatov, A. V., Govindaswami, M., & Brown, S. A. (2009). Activation of peripheral δ2 opioid receptors increases cardiac tolerance to ischemia/reperfusion injury: Involvement of protein kinase C, NO-synthase, KATP channels and the autonomic nervous system. Life Sciences, 84, 657–663.
Mahmoud, S., Gharagozloo, M., Simard, C., & Gris, D. (2019). Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells, 8(2), 184.
Mayanagi, K., Gáspár, T., Katakam, P. V., Kis, B., & Busija, D. W. (2007). The mitochondrial K(ATP) channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism, 27(2), 348–355.
Mironova, G. D., Shigaeva, M. I., Gritsenko, E. N., Murzaeva, S. V., Gorbacheva, O. S., Germanova, E. L., & Lukyanova, L. D. (2010). Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal’s adaptation to hypoxia. Journal of Bioenergetics and Biomembranes, 42(6), 473–481.
Neckár, J., Papousek, F., Nováková, O., Ost’ádal, B., & Kolár, F. (2002). Cardioprotective effects of chronic hypoxia and ischaemic preconditioning are not additive. Basic Research in Cardiology, 97(2), 161–167.
Obrenovitch, T. P. (2008). Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiological Reviews, 88, 211–247.
Pevsner, P. H., Eichenbaum, J. W., Miller, D. C., Pivawer, G., Eichenbaum, K. D., Stern, A., Zakian, K. L., & Koutcher, J. A. (2001). A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. Journal of Pharmacological and Toxicological Methods, 45, 227–233.
Pruimboom, L., & Muskiet, F. A. J. (2018). Intermittent living: The use of ancient challenges as a vaccine against the deleterious effects of modern life—A hypothesis. Medical Hypotheses, 120, 28–42.
Qi, L., Meng, L., Li, Y., & Qu, Y. (2012). Arterial carbon dioxide partial pressure influences CYP4A distribution in the rat brain. Histology and Histopathology, 27(7), 897–903.
Rajapakse, N., Kis, B., Horiguchi, T., Snipes, J., & Busija, D. (2003). Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. Journal of Neuroscience Research, 73, 206–214.
Rebola, N., Pinheiro, P. C., Oliveira, C. R., Malva, J. O., & Cunha, R. A. (2003). Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Research, 987(1), 49–58.
Rybnikova, E., & Samoilov, M. (2015). Current insights into the molecular mechanisms of hypoxic pre- and postconditioning using hypobaric hypoxia. Frontiers in Neuroscience, 9, 388.
Sasaki, N., Sato, T., Ohler, A., O’Rourke, B., & Marban, E. (2000). Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation, 101, 439–445.
Stockwell, J., Jakova, E., & Cayabyab, F. S. (2017). Adenosine A1 and A2A receptors in the brain: Current research and their role in neurodegeneration. Molecules, 22(4), 676.
Smith, C. O., Nehrke, K., & Brookes, P. S. (2017). The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection. The Biochemical Journal, 474(12), 2067–2094.
Sharp, F. R., Ran, R., Lu, A., Tang, Y., Strauss, K. I., Glass, T., Ardizzone, T., & Bernaudin, M. (2004). Hypoxic preconditioning protects against ischemic brain injury. NeuroRx, 1(1), 26–35.
Shatilo, V. B., Korkushko, O. V., Ischuk, V. A., Downey, H. F., & Serebrovskaya, T. V. (2008). Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Altitude Medicine & Biology, 9(1), 43–52.
Siafakas, N. M., Jordan, M., Wagner, H., Breen, E. C., Benoit, H., & Wagner, P. D. (2001). Diaphragmatic angiogenic growth factor mRNA responses to increased ventilation caused by hypoxia and hypercapnia. European Respiratory Journal, 17, 681–687.
Sun, X. L., Zeng, X. N., Zhou, F., Dai, C. P., Ding, J. H., & Hu, G. (2008). KATP channel openers facilitate glutamate uptake by gluts in rat primary cultured astrocytes. Neuropsychopharmacology, 33, 1336–1342.
Sun, J., Tong, L., Luan, Q., Deng, J., Li, Y., Li, Z., Dong, H., & Xiong, L. (2012). Protective effect of delayed remote limb ischemic postconditioning: Role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. Journal of Cerebral Blood Flow and Metabolism, 32(5), 851–859.
Szeto, V., Chen, N. H., Sun, H. S., & Feng, Z. P. (2018). The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacologica Sinica, 39(5), 683–694.
Tao, T., Liu, Y., Zhang, J., Xu, Y., Li, W., & Zhao, M. (2013). Therapeutic hypercapnia improves functional recovery and attenuates injury via antiapoptotic mechanisms in a rat focal cerebral ischemia/reperfusion model. Brain Research, 1533, 52–62.
Tetzlaff, W., Schubert, P., & Kreutzberg, G. W. (1987). Synaptic and extrasynaptic localization of adenosine binding sites in the rat hippocampus. Neuroscience, 21(3), 869–875.
Tregub, P., Kulikov, V., & Bespalov, A. (2013). Tolerance to acute hypoxia maximally increases in case of joint effect of normobaric hypoxia and permissive hypercapnia in rats. Pathophysiology, 3, 165–170.
Tregub, P. P., Kulikov, V. P., Stepanova, L. A., Zabrodina, A. S., & Nagibaeva, M. E. (2014). The role of adenosine A1 receptors and mitochondrial K+ATP channels in the mechanism of increasing the resistance to acute hypoxia in the combined effects of hypoxia and hypercapnia. Patologicheskaia Fiziologiia i Eksperimentalnaia Terapiia, 4, 48–52.
Tregub, P., Kulikov, V., Motin, Y., Bespalov, A., & Osipov, I. (2015). Combined exposure to hypercapnia and hypoxia provides its maximum neuroprotection effect during focal ischemic injury in the brain. Journal of Stroke and Cerebrovascular Diseases, 24(2), 381–387.
Wang, L., Zhu, Q. L., Wang, G. Z., Deng, T. Z., Chen, R., Liu, M. H., & Wang, S. W. (2011). The protective roles of mitochondrial ATP-sensitive potassium channels during hypoxia-ischemia-reperfusion in brain. Neuroscience Letters, 491(1), 63–67.
Yang, C. C., Lin, L. C., Wu, M. S., Chien, C. T., & Lai, M. K. (2009). Repetitive hypoxic preconditioning attenuates renal ischemia/reperfusion induced oxidative injury via upregulating HIF-1 alpha-dependent bcl-2 signaling. Transplantation, 88, 1251–1260.
Yellon, D. M., & Downey, J. M. (2003). Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiological Reviews, 83(4), 1113–1151.
Zakynthinos, S., Katsaounou, P., Karatza, M. H., Roussos, C., & Vassilakopoulos, T. (2007). Antioxidants increase the ventilatory response to hyperoxic hypercapnia. American Journal of Respiratory and Critical Care Medicine, 175, 62–68.
Zhang, D., Chen, Y., Campbell, W., Zou, A., Gross, G., & Li, P. (2001). Characteristics and suproxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channel. Circulation Research, 89, 1177–1183.
Zhao, Z. S., Khan, S., & O’Brien, P. J. (1998). Catecholic iron complexes as cytoprotective superoxide scavengers against hypoxia/reoxygenation injury in isolated hepatocytes. Biochemical Pharmacology, 56(7), 825–830.
Zhao, Y. D., Cheng, S. Y., Ou, S., Xiao, Z., He, W. J., Jian-Cui, & Ruan, H. Z. (2012). Effect of hypobaric hypoxia on the P2X receptors of pyramidal cells in the immature rat hippocampus CA1 sub-field. Brain Injury, 26(3), 282–290.
Zhou, Q., Cao, B., Niu, L., Cui, X., Yu, H., Liu, J., Li, H., & Li, W. (2010). Effects of permissive hypercapnia on transient global cerebral ischemia–reperfusion injury in rats. Anesthesiology, 112, 288–297.
Acknowledgements
The study was supported by a grant from the Russian Science Foundation (Project No. 18-75-00016). We wish to thank Prof. Alla Salmina and Dr. Elizaveta Boytsova for great help in the work on this study.
Funding
The study was supported by a grant from the Russian Science Foundation (Project No. 18-75-00016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Animal experiments were approved by the Bioethical Commission of the local Ethics Committee of Krasnoyarsk State Medical University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (EU Directive 2010/63/EU for animal experiments).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tregub, P.P., Malinovskaya, N.A., Osipova, E.D. et al. Hypercapnia Modulates the Activity of Adenosine A1 Receptors and mitoK+ATP-Channels in Rat Brain When Exposed to Intermittent Hypoxia. Neuromol Med 24, 155–168 (2022). https://doi.org/10.1007/s12017-021-08672-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-021-08672-0