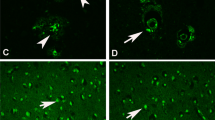
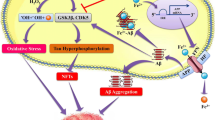
Abstract
Metal dyshomeostasis in the brain (BMD) has often been proposed as a possible cause for several neurodegenerative disorders (NDs). Nevertheless, the precise nature of the biochemical mechanisms of metal involvement in NDs is still largely unknown. Mounting evidence suggests that normal aging itself is characterized by, among other features, a significant degree of metal ion dysmetabolism in the brain. This is probably the result of a progressive deterioration of the metal regulatory systems and, at least in some cases, of life-long metal exposure and brain accumulation. Although alterations of metal metabolism do occur to some extent in normal aging, they appear to be highly enhanced under various neuropathological conditions, causing increased oxidative stress and favoring abnormal metal–protein interactions. Intriguingly, despite the fact that most common NDs have a distinct etiological basis, they share striking similarities as they are all characterized by a documented brain metal impairment. This review will primarily focus on the alterations of metal homeostasis that are observed in normal aging and in Alzheimer’s disease. We also present a brief survey on BMD in other NDs (Amyotrophic Lateral Sclerosis, Parkinson’s, and Prion Protein disease) in order to highlight what represents the most reliable evidence supporting a crucial involvement of metals in neurodegeneration. The opportunities for metal-targeted pharmacological strategies in the major NDs are briefly outlined as well.






Similar content being viewed by others
References
Adlard, P. A., Cherny, R. A., Finkelstein, D. I., Gautier, E., Robb, E., Cortes, M., et al. (2008). Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron, 59, 43–55.
Age-Related Eye Disease Study Research Group. (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology, 119, 1417–1436.
Alfrey, A. C., LeGendre, G. R., & Kaehny, W. D. (1976). The dialysis encephalopathy syndrome. Possible aluminum intoxication. New England Journal of Medicine, 294, 184–188.
Alimonti, A., Ristori, G., Giubilei, F., Stazi, M. A., Pino, A., Visconti, A., et al. (2007). Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology, 28, 450–456.
Allsop, D., Mayes, J., Moore, S., Masad, A., & Tabner, B. J. (2008). Metal-dependent generation of reactive oxygen species from amyloid proteins implicated in neurodegenerative disease. Biochemical Society Transactions, 36, 1293–1298.
Altamura, S., & Muckenthaler, M. U. (2009). Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. Journal of Alzheimers Diseases, 16, 879–895.
Andersen, J. K. (2004). Iron dysregulation and Parkinson’s disease. Journal of Alzheimers Diseases, 6, 47–52.
Babiloni, C., Squitti, R., Del Percio, C., Cassetta, E., Ventriglia, M. C., Ferreri, F., et al. (2007). Free copper and restino temporal EEG rhythms correlate across healthy, mild cognitive impairment, and Alzheimer’s disease subjects. Clinical Neurophysiology, 118, 1244–1260.
Bala Gupta, V., Anitha, S., Hedge, M. L., Zecca, L., Garruto, M. R., Ravid, R., et al. (2005). Aluminium in Alzheimer’s disease: Are we still at a crossroad? Cellular and Molecular Life Sciences, 62, 143–158.
Banks, W. A., Niehoff, M. L., Drago, D., & Zatta, P. (2006). Aluminum complexing enhances amyloid beta protein penetration of blood-brain barrier. Brain Research, 1116, 215–221.
Barnham, K. J., & Bush, A. I. (2008). Metals in Alzheimer’s and Parkinson’s diseases. Current Opinion in Chemical Biology, 12, 222–228.
Bayer, T. A., Schäfer, S., Breyhan, H., Wirths, O., Treiber, C., & Multhaup, G. (2006). A vicious circle: Role of oxidative stress, intraneuronal Abeta and Cu in Alzheimer’s disease. Clinical Neuropathology, 25, 163–171.
Bayer, T. A., Schäfer, S., Simons, A., Kemmling, A., Kamer, T., Tepest, R., et al. (2003). Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America, 100, 14187–14192.
Bayir, H., Kapralov, A. A., Jiang, J., Huang, Z., Tyurina, Y. Y., Tyurin, V. A., et al. (2009). Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome c: Protection agains apoptosis versus delayed oxidative stress in Parkinson’s disease. Journal of Biological Chemistry, 284, 15951–15961.
Bertoni-Freddari, C., Fattoretti, P., Casoli, T., Di Stefano, G., Giorgetti, B., & Balietti, M. (2008). Brain aging: The zinc connection. Experimental Gerontology, 43, 389–393.
Bertoni-Freddari, C., Fattoretti, P., Paoloni, R., Caselli, U., Galeazzi, L., & Meier-Ruge, W. (1996). Synaptic structural dynamics and aging. Gerontology, 42, 170–180.
Binolfi, A., Lamberto, G. R., Duran, R., Quintanar, L., Bertoncini, C. W., Souza, J. M., et al. (2008). Site-specific interactions of Cu(II) with α and β-synuclein: Bridging the molecular gap between metal binding and aggregation. Journal of the American Chemical Society, 130, 11801–11812.
Boillée, S., Vande Velde, C., & Cleveland, D. W. (2006). ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron, 52, 39–59.
Bolognin, S., Drago, D., Messori, L., & Zatta, P. (2009). Chelation therapy for neurodegenerative diseases. Medicinal Research Reviews, 29, 547–570.
Bolognin, S., Zatta, P., Drago, D., Parnigotto, P. P., Tognon, G., & Ricchelli, F. (2008). Mutual stimulation of beta-amyloid fibrillogenesis by clioquinol and divalent metals. Neuromolecular Medicine, 10, 322–332.
Borchardt, T., Camakaris, J., Cappai, R., Master, C. L., Beyreuther, K., & Multhaup, G. (1999). Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor protein secretion. Biochemical Journal, 344, 461–467.
Bouras, C., Giannakopoulos, P., Good, P. F., Hsu, A., Hof, P. R., & Perl, D. P. (1997). A laser microprobe mass analysis of brain aluminum and iron in dementia pugilistica: Comparison with Alzheimer’s disease. European Neurology, 38, 53–58.
Brown, D. R. (2009). Brain proteins that mind metals: A neurodegenerative perspective. Dalton Transactions, 21, 4069–4076.
Brown, D. R., Hafiz, F., Glassmith, L. L., Wong, B. S., Jones, I. M., Clive, C., et al. (2000). Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO Journal, 19, 1180–1186.
Brown, D. R., Wong, B. S., Hafiz, F., Clive, C., Haswell, S. J., & Jones, I. M. (1999). Normal prion protein has an activity like that of superoxide dismutase. Biochemical Journal, 344, 1–5.
Bush, A. I. (2003). The metallobiology of Alzheimer’s disease. Trends in Neurosciences, 26, 207–214.
Butterfield, D. A., Reed, T., Newman, S. F., & Sultan, R. (2007). Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radical Biology and Medicine, 43, 658–677.
Cahill, C. M., Lahiri, D. K., Huang, X., & Rogers, J. T. (2009). Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochimica et Biophysica Acta, 1790, 615–628.
Campbell, A. (2006). The role of aluminum and copper on neuroinflammation and Alzheimer’s disease. Journal of Alzheimers Diseases, 10, 165–172.
Cherny, R. A., Atwood, C. S., Xilinas, M. E., Gray, D. N., Jones, W. D., Mclean, C. A., et al. (2001). Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron, 30, 665–676.
Connor, J. R., & Lee, S. Y. (2006). HFE mutations and Alzheimer’s disease. Journal of Alzheimers Diseases, 10, 267–276.
Crichton, R. R., Dexter, D. T., & Roberta, J. W. (2008). Metal based neurodegenerative diseases—from molecular mechanisms to therapeutic strategies. Coordination Chemistry Reviews, 251, 1189–1199.
Cross, D. J., Flexman, J. A., Anzai, Y., Morrow, T. J., Maravilla, K. R., & Minoshima, S. (2006). In vivo imaging of functional disruption, recovery and alteration in rat olfactory circuitry after lesion. Neuroimage, 32, 1265–1272.
Crouch, P. J., Barnham, K. J., Bush, A. I., & White, A. R. (2006). Therapeutic treatments for Alzheimer’s disease based on metal bioavailability. Drug News Perspective, 19, 469–474.
Crouch, P. J., Hung, L. W., Adlard, P. A., Cortes, M., Lal, V., Filiz, G., et al. (2009). Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proceedings of the National Academy of Sciences of the United States of America, 106, 381–386.
Crow, P., Sampson, J. B., Zhuang, Y., Thompson, J. A., & Beckman, J. S. (1997). Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. Journal of Neurochemistry, 69, 1936–1944.
Cuajungco, M. P., & Faget, K. Y. (2003). Zinc takes the center stage: Its paradoxical role in Alzheimer’s disease. Brain Research Reviews, 41, 44–56.
Cuajungco, M. P., & Lees, G. J. (1997). Zinc and Alzheimer’s disease: Is there a direct link? Brain Research Reviews, 23, 219–236.
Deibel, M. A., Ehmann, W. D., & Markesbery, W. R. (1996). Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: Possible relation to oxidative stress. Journal of the Neurological Sciences, 143, 137–142.
Del Corso, L., Pastine, F., Protti, M. A., Romanelli, A. M., Moruzzo, D., Rocco, L., et al. (2000). Blood zinc, copper and magnesium in ageing. A study in healthy home-living elderly. Panminerva Medica, 42, 273–277.
Deshpande, A., Kawai, H., Metherate, R., Glabe, C. G., & Busciglio, J. (2009). A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. Journal of Neuroscience, 29, 4004–4015.
Dexter, D. T., Carayon, A., Javoy-Agid, F., Agid, Y., Wells, F. R., Daniel, S. E., et al. (1991). Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain, 114, 1953–1975.
Dong, J., Robertson, J. D., Merkesbery, W. R., & Lovell, M. A. (2008). Serum zinc in the progression of Alzheimer’s Disease. Journal of Alzheimers Diseases, 15, 443–450.
Drago, D., Bettella, M., Bolognin, S., Cendron, L., Scancar, J., Milacic, R., et al. (2008a). Potential pathogenic role of beta-amyloid(1–42)-aluminum complex in Alzheimer’s disease. International Journal of Biochemistry and Cell Biology, 40, 731–746.
Drago, D., Bolognin, S., & Zatta, P. (2008b). Role of metal ions in the abeta oligomerization in Alzheimer’s disease and in other neurological disorders. Current Alzheimer Research, 5, 5007–5507.
Drago, D., Cavaliere, A., Mascetra, N., Ciavardelli, D., di Ilio, C., Zatta, P., et al. (2008c). Aluminum modulates effects of beta amyloid(1–42) on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer’s disease. Rejuvenation Resarch, 11, 861–871.
Eisen, A. (2009). Amyotrophic lateral sclerosis: A 40-year personal perspective. Journal of Clinical Neuroscience, 16, 505–512.
Estevez, A. G., Crow, J. P., Sampson, J. B., Reiter, C., Zhuang, Y., Richardson, G. J., et al. (1999). Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science, 286, 2498–2500.
Faucheux, B. A., Martin, M. E., Beaumont, C., Hauw, J. J., Agid, Y., & Hirsch, E. C. (2003). Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. Journal of Neurochemistry, 86, 1142–1148.
Ferrer, M., Golyshina, O. V., Beloqui, A., Golyshin, P. N., & Timmis, K. N. (2007). The cellular machinery of Ferroplasma acidiphilum is iron-protein-dominated. Nature, 445, 91–94.
Frederickson, C. J., Koh, J. Y., & Bush, A. I. (2005). The neurobiology of zinc in health and disease. Nature Reviews Neuroscience, 6, 449–462.
Garrick, M. D., & Garrick, L. M. (2009). Cellular iron transport. Biochimica et Biophysica Acta, 1790, 309–325.
Gerhardsson, L., Lundh, T., Minthon, L., & Londos, E. (2008). Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 25, 508–515.
Glabe, C. C. (2005). Amyloid accumulation and pathogensis of Alzheimer’s disease: Significance of monomeric, oligomeric and fibrillar Abeta. SubCellular Biochemistry, 38, 167–177.
Good, P. F., & Perl, D. P. (1993). Aluminium in Alzheimer’s? Nature, 362, 418.
Goodall, E. F., Haque, M. S., & Morrison, K. E. (2008). Increased serum ferritin levels in amyotrophic lateral sclerosis (ALS) patients. Journal of Neurology, 255, 1652–1656.
Goto, J. J., Zhu, H., Sanchez, R. J., Nersissian, A., Gralla, E. B., Valentie, J. S., et al. (2000). Loss of in vitro metal ion binding specificity in mutant copper-zinc superoxide dismutases associated with familial amyotrophic lateral sclerosis. Journal of Biological Chemistry, 275, 1007–1014.
Gotz, M. E., Double, K., Gerlach, M., Youdim, M. B., & Riederer, P. (2004). The relevance of iron in the pathogenesis of Parkinson’s disease. Annals of the New York Academy of Sciences, 1012, 193–208.
Haass, C., & Selkoe, D. J. (2007). Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nature Reviews Molecular Cell Biology, 8, 101–112.
Harris, D. A., & True, H. L. (2006). New insights into prion structure and toxicity. Neuron, 40, 547–586.
Hegde, M. L., Bharathi, P., Suram, A., Venugopal, C., Jagannathan, R., Poddar, P., et al. (2009). Challenges associated with metal chelation therapy in Alzheimer’s disease. Journal of Alzheimers Diseases (in press).
Hesketh, S., Sassoon, J., Knight, R., & Brown, D. R. J. (2008). Elevated manganese levels in blood and CNS in human prion disease. Molecular and Cellular Neurosciences, 37, 590–598.
Hesketh, S., Sassoon, J., Knight, R., Hopkins, J., & Brown, D. R. J. (2007). Elevated manganese levels in blood and central nervous system occur before onset of clinical signs of scrapie and bovine spongiform encephalopathy. Journal of Animal Science, 85, 1596–1609.
Hirsch, E. C., Brandel, J. P., Galle, P., Javoy-Agid, F., & Agid, Y. (1991). Iron and aluminum in the substantia nigra of patients with Parkinson’s disease: An X-ray microanalysis. Journal of Neurochemistry, 56, 446–451.
Hu, W. P., Chang, G. L., Chen, S. J., & Kuo, Y. M. (2006). Kinetic analysis of beta-amyloid peptide aggregation induced by metal ions based on surface plasmon resonance biosensing. Journal of Neuroscience Methods, 154, 190–197.
Hwang, E. M., Kim, S. K., Sohn, J. H., Lee, J. Y., Kim, Y., Kim, Y. S., et al. (2006). Furin is an endogenous regulator of alpha-secretase associated APP processing. Biochemical and Biophysical Research Communications, 349, 654–659.
Iqbal, K., Liu, F., Gong, C. X., Alonso Adel, C., & Grundke-Iqbal, I. (2009). Mechanisms of tau-induced neurodegeneration. Acta Neuropathologica, 118, 53–69.
Jellinger, K. A. (1999). The role of iron in neurodegeneration: Prospects for pharmacotherapy of Parkinson’s disease. Drugs and Aging, 14, 115–140.
Jellinger, K. A. (2003). General aspects of neurodegeneration. Journal of Neural Transmission (Supplementum), 65, 101–144.
Jeong, S. Y., Rathore, K. I., Schulz, K., Ponka, P., Arosio, P., & David, S. (2009). Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. Journal of Neuroscience, 29, 610–619.
Kasarskis, E. J., Tandon, L., Lovell, M. A., & Ehmann, W. D. (1995). Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: A preliminary study. Journal of the Neurological Sciences, 130, 203–208.
Kaur, D., & Andersen, J. K. (2002). Ironing out Parkinson’s disease: Is therapeutic treatment with iron chelators a real possibility? Aging Cell, 1, 17–21.
Kawarabayashi, T., Shoji, M., Younkin, L. H., Wen-Lang, L., Dickson, D. W., Murakami, T., et al. (2004). Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. Journal of Neuroscience, 24, 3801–3809.
Kenward, A. G., Bartolotti, L. J., & Burns, C. S. (2007). Copper and zinc promote interactions between membrane-anchored peptides of the metal binding domain of the prion protein. Biochemistry, 46, 4261–4271.
Kessler, H., Bayer, T. A., Bach, D., Schneider-Axmann, T., Supprian, T., Herrmann, W., et al. (2008). Intake of copper has no effect on cognition in patients with mild Alzheimer’s disease: A pilot phase 2 clinical trial. Journal of Neural Transmission, 115, 1181–1187.
Kim, Y., Lee, J. H., Ryu, J., & Kim, D. J. (2009). Multivalent & multifunctional ligands to beta-amyloid. Current Pharmaceutical Design, 15, 637–658.
Kitazawa, M., Cheng, D., & LaFerla, F. M. (2009). Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. Journal of Neurochemistry, 108, 1550–1560.
Konoho, K., Sadakane, Y., & Kawahara, M. (2006). Zinc neurotoxicity and its role in neurodegenerative diseases. Journal of Health Sciences, 52, 1–8.
Kramer, D. R., Llanos, R. M., & Mercer, J. F. B. (2003). Molecular basis of copper transport: Cellular and physiological functions of Menkes and Wilson disease proteins. In Metal ions and neurodegenerative disorders (pp. 183–206). Singapore, London: World Scientific.
Krewski, D., Yokel, R. A., Nieboer, E., Borchelt, D., Cohen, J., Harry, J., et al. (2007). Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. Journal of Toxicology and Environmental Health B: Critical Review, 10, 1–269.
Laurén, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W., & Strittmatter, S. M. (2009). Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature, 457, 1128–1132.
Lee, J. Y., Cole, T. B., Palmiter, R. D., Sush, S. W., & Koh, J. Y. (2002). Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proceedings of the National Academy of Sciences of the United States of America, 99, 7705–7710.
Lee, J. Y., Friedman, J. E., Angel, I., Kozak, A., & Koh, J. Y. (2004). The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiology of Aging, 25, 1315–1321.
Leong, S. L., Pham, C. L., Galatis, D., Fodero-Tavoletti, M. T., Perez, K., Hill, A. F., et al. (2009). Formation of dopamine-mediated alpha-synuclein-soluble oligomers requires methionine oxidation. Free Radical Biology and Medicine, 46, 1328–1337.
Lewczuk, P., Esselmann, H., Otto, M., Maler, J. M., Henkel, A. W., Henkel, M. K., et al. (2004). Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiology of Aging, 25, 273–281.
Linkous, D. H., Adlard, P. A., Wanschura, P. B., Conko, K. M., & Flinn, J. M. (2009). The effects of enhanced Zinc on spatial memory and plaque formation in transgenic mice. Journal of Alzheimers Diseases (in press).
Liu, G., Garrett, M. R., Men, P., Zhu, X., Perry, G., & Smith, M. A. (2005). Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochimica et Biophysica Acta, 1741, 246–252.
Liu, G., Men, P., Harris, P. L., Rolston, R. K., Perry, G., & Smith, M. A. (2006). Nanoparticle iron chelators: A new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neuroscience Letters, 406, 189–193.
Loeffler, D. A., LeWitt, P. A., Juneau, P. L., Sima, A. A., Nguyen, H. U., DeMaggio, A. J., et al. (1996). Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Research, 738, 265–274.
Lovell, M. A. (2009). A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer’s disease. Journal of Alzheimers Diseases, 16, 471–483.
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L., & Markesbery, W. R. (1998). Copper, iron and zinc in Alzheimer’s disease senile plaques. Journal of the Neurological Sciences, 158, 47–52.
Lu, J., Zheng, Y. L., Wu, D. M., Sun, D. X., Shan, Q., & Fan, S. H. (2006). Trace amount of copper induce neurotoxicity in the cholesterol-fed mice through apoptosis. FEBS Letters, 580, 6730–6740.
Lyons, T. J., Liu, H., Goto, J. J., Nersissian, A., Roe, J. A., Graden, J. A., et al. (1996). Mutations in copper-zinc superoxide dismutase that cause amyotrophic lateral sclerosis alter the zinc binding site and the redox behavior of the protein. Proceedings of the National Academy of Sciences of the United States of America, 93, 12240–12244.
Miller, L. M., Wang, Q., Telivala, T. P., Smith, R. J., Lanzirotti, A., & Miklossy, J. (2006). Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. Journal of Structural Biology, 155, 30–37.
Miura, T., Hori-i, A., & Takeuchi, H. (1996). Metal-dependent a-helix formation promoted by the glycine-rich octapeptide region of prion protein. FEBS Letters, 396, 248–252.
Mocchegiani, E., Bertoni-Freddari, C., Marcellini, F., & Malavolta, M. (2005). Brain, aging, and neurodegeneration: Role of zinc availability. Progress in Neurobiology, 75, 367–390.
Molina, J. A., Jiménez-Jiménez, F. J., Aguilar, M. V., Meseguer, I., Mateos-Vega, C. J., González-Muñoz, M. J., et al. (1998). Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease. Journal of Neural Transmission, 105, 479–488.
Morita, A., Kimura, M., & Itokawa, Y. (1994). The effect of aging on the mineral status of female mice. Biological Trace Element Research, 42, 165–177.
Morris, M. C., Evans, D. A., Tangney, C. C., Bienias, J. L., Schneider, J. A., Wilson, R. S., et al. (2006). Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Archives of Neurology, 63, 1085–1088.
Nakashima, A. S., & Dyck, R. H. (2009). Zinc and cortical plasticity. Brain Research Reviews, 59, 347–373.
Nicolas, O., Gavín, R., & Del Río, J. A. (2009). New insights into cellular prion protein (PrP(c)) functions: The “ying and yang” of a relevant protein. Brain Research Reviews (in press).
Olivares, D., Huang, X., Branden, L., Greig, N. H., & Rogers, J. T. (2009). Physiological and pathological role of alpha-synuclein in Parkinson’s disease through iron mediated oxidative stress; the role of a putative iron-responsive element. International of Journal of Molecular Science, 10, 1226–1260.
Opazo, C., Huang, X., Cherny, R. A., Moir, R. D., Roher, A. E., White, A. R., et al. (2002). Metalloenzyme-like activity of Alzheimer’s disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). Journal of Biological Chemistry, 277, 40302–40308.
Osterova-Golts, N., Petrucelli, L., Hardy, J., Lee, J. M., Farer, M., & Wolozin, B. (2000). The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. Journal of Neuroscience, 20, 6048–6054.
Oztug Durer, Z. A., Cohlberg, J. A., Dinh, P., Padua, S., Ehrenclou, K., Downes, S., et al. (2009). Loss of metal ions, disulfide reduction and mutations related to familial ALS promote formation of amyloid-like aggregates from superoxide dismutase. PLoS ONE, 4, e5004.
Paik, S. R., Lee, J. H., Kim, D. H., Chang, C. S., & Kim, J. (1997). Aluminum-induced structural alterations of the precursor of the non-A beta component of Alzheimer’s disease amyloid. Archives of Biochemistry and Biophysics, 344, 325–334.
Pantopoulos, K., & Hentze, M. W. (1998). Activation of iron regulatory protein-1 by oxidative stress in vitro. Proceedings of the National Academy of Sciences of the United States of America, 95, 10559–10563.
Petri, S., Calingasan, N. Y., Alsaied, O. A., Wille, E., Kiaei, M., Friedman, J. E., et al. (2007). The lipophilic metal chelators DP-109 and DP-460 are neuroprotective in a transgenic mouse model of amyotrophic lateral sclerosis. Journal of Neurochemistry, 102, 991–1000.
Phinney, A. L., Drisaldi, B., Schmidt, S. D., Lugowski, S., Coronado, V., Liang, Y., et al. (2003). In vivo reduction of amyloid-beta by a mutant copper transporter. Proceedings of the National Academy of Sciences of the United States of America, 100, 14193–14198.
Powers, K. M., Smith-Weller, T., Franklin, G. M., Longstreth, W. T., Jr, Swanson, P. D., & Checkoway, H. (2003). Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology, 60, 1761–1766.
Prusiner, S. B. (1998). Prions. Proceedings of the National Academy of Sciences of the United States of America, 95, 13363–13383.
Pushie, M. J., Rauk, A., Jirik, F. R., & Vogel, H. J. (2009). Can copper binding to the prion protein generate a misfolded form of the protein. BioMetals, 22, 159–179.
Quinn, J. F., Crane, S., Harris, C., & Wadsworth, T. L. (2009). Copper in Alzheimer’s disease: Too much or too little? Expert Review of Neurotherapeutics, 9, 631–637.
Rachidi, W., Vilette, D., Guiraud, P., Arlotto, M., Riondel, J., Laude, H., et al. (2003). Expression of prion protein increases cellular copper binding and antioxidant enzyme activities but not copper delivery. Journal of Biological Chemistry, 278, 9064–9072.
Rajan, M. T., Jagannatha Rao, K. S., Mamatha, B. M., Rao, R. V., Shanmugavelu, P., Menon, R. B., et al. (1997). Quantification of trace elements in normal human brain by inductively coupled plasma atomic emission spectrometry. Journal of the Neurological Sciences, 146, 153–166.
Rajendran, R., Minqin, R., Ynsa, M. D., Casadesus, G., Smith, M. A., Perry, G., et al. (2009). A novel approach to the identification and quantitative elemental analysis of amyloid deposits insights into the pathology of Alzheimer’s disease. Biochemical and Biophysical Research Communications, 382, 91–95.
Re, L., Rossini, F., Re, F., Bordicchia, M., Mercanti, A., Fernandez, O. S., et al. (2006). Prion protein potentiates acetylcholine release at the neuromuscular junction. Pharmacological Research, 53, 62–68.
Reusche, E. (2003). Aluminium and central nervous system morphology in hemodialysis. In Metal ions and neurodegenerative disorders (pp. 117–138). Singapore, London: World Scientific.
Ricchelli, F., Buggio, R., Drago, D., Forloni, G., Negro, A., Tognon, G., et al. (2006). Aggregation/Fibrillogenesis of recombinant human prion protein and Gestmann-Straussler-Scheinker disease peptides in the presence of metal ions. Biochemistry, 45, 6724–6732.
Ricchelli, F., Drago, D., Filippi, B., Tognon, G., & Zatta, P. (2005). Aluminum-triggered structural modifications and aggregation of beta-amyloids. Cellular and Molecular Life Sciences, 62, 1724–1733.
Ricchelli, F., Fusi, P., Tortora, P., Valtorta, M., Riva, M., Tognon, G., et al. (2007). Destabilization of non-pathological variants of ataxin-3 by metal ions results in aggregation/fibrillogenesis. International Journal of Biochemistry and Cell Biology, 39, 966–977.
Ritchie, C. W., Bush, A. I., Mackinnon, A., Macfarlane, S., Mastwyk, M., MacGregor, L., et al. (2003). Metal-protein attenuation with clioquinol targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Archives of Neurology, 60, 1685–1691.
Rogers, J. T., Randall, J. D., Cahill, C. M., Eder, P. S., Huang, X., Gunshin, H., et al. (2002a). An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. Journal of Biological Chemistry, 277, 45518–45528.
Rogers, J. T., Randall, J. D., Eder, P. S., Huang, X., Bush, A. I., Tanzi, R. E., et al. (2002b). Alzheimer’s disease drug discovery targeted to the APP mRNA 5′ untranslated region. Journal of Molecular Neuroscience, 19, 77–82.
Roider, G., & Drasch, G. (1999). Concentration of Al in human tissues. Investigations on an occupationally non-exposed population in Southern Bavaria (Germany). Trace Elements Electrolytes, 16, 77–86.
Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Snapp, P., Hentati, A., et al. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature, 362, 59–62.
Rulon, L. L., Robertson, J. D., Lovell, M. A., Deibel, M. A., Ehmann, W. D., & Markesber, W. R. (2000). Serum zinc levels and Alzheimer’s disease. Biological Trace Element Research, 75, 79–85.
Said Ahmed, M., Hung, W. Y., Zu, J. S., Hockberger, P., & Siddique, T. (2000). Increased reactive oxygen species in familial amyotrophic lateral sclerosis with mutations in SOD1. Journal of the Neurological Sciences, 176, 88–94.
Salzman, M. B., Smith, E. M., & Koo, C. (2002). Excessive oral zinc supplementation. Journal of Pediatric Hematology/oncology, 24, 582–584.
Sassoon, J., & Brown, D. R. (2003). Copper and prion disease. In Metal ions and neurodegenerative disorders (pp. 279–306). Singapore, London: World Scientific.
Sayre, L. M., Moreira, P. A., Smith, M. A., & Perry, G. (2005). Metal ions and oxidative protein modification in neurological disease. Annals of Ist Super Sanità, 41, 143–164.
Schipper, H. M. (2004). Heme oxygenase expression in human central nervous system disorders. Free Radical Biology and Medicine, 37, 1995–2011.
Sigurdsson, E. M., Brown, D. R., Alim, M. A., Scholtzova, H., Carp, R., Meeker, H. C., et al. (2003). Copper chelation delays the onset o prion disease. Journal of Biological Chemistry, 278, 46199–46202.
Silvestri, L., & Camaschella, C. (2008). A potential pathogenetic role of iron in Alzheimer’s disease. Journal of Cellular and Molecular Medicine, 12, 1548–1550.
Smith, D. G., Cappai, R., & Barnham, K. J. (2007). The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochimica et Biophysica Acta, 1768, 1976–1990.
Sofic, E., Sapcanin, A., Tahirovic, I., Gavrankapetanovic, I., Jellinger, K., Reynolds, G. P., et al. (2006). Antioxidant capacity in post-mortem brain tissues of Parkinson’s and Alzheimer’s diseases. Journal of Neural Transmission (Supplementum), 71, 39–43.
Soto, C., & Estrada, L. D. (2008). Protein misfolding and neurodegeneration. Archives of Neurology, 65, 184–189.
Sparks, D. L., & Schreurs, B. G. (2003). Trace amount of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 100, 11065–11069.
Speziali, M., & Orvini, E. (2003). Metals distribution and regionalization in the brain. In Metal ions and neurodegenerative disorders (pp. 15–65). Singapore, London: World Scientific.
Squitti, R., Barbati, G., Rossi, L., Ventriglia, M., Dal Forno, G., Cesaretti, S., et al. (2006). Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology, 67, 76–82.
Squitti, R., Cassetta, E., Dal Forno, G., Lupoi, D., Lippolis, G., Pauri, F., et al. (2004). Copper perturbation in 2 monozygotic twins discordant for degree of cognitive impairment. Archives of Neurology, 61, 738–743.
Squitti, R., Pasqualetti, P., Dal Forno, G., Moffa, F., Cassetta, E., Lupoi, D., et al. (2005). Excess of serum copper not related to ceruloplasmin in Alzheimer disease. Neurology, 64, 1040–1046.
Srivastava, R. A., & Jain, J. C. (2002). Scavenger receptor class B type I expression and elemental analysis in cerebellum and parietal cortex regions of the Alzheimer’s disease brain. Journal of the Neurological Sciences, 196, 45–52.
Stankiewicz, J. M., & Brass, S. D. (2009). Role of iron in neurotoxicity: A cause for concern in the elderly? Current Opinion in Clinical Nutrition and Metabolic Care, 12, 22–29.
Stoltenberg, M., Bruhn, M., Sondergaard, C., Doering, P., West, M. J., Larsen, A., et al. (2005). Immersion autometallographic tracing of zinc ions in Alzheimer beta-amyloid plaques. Histochemistry and Cell Biology, 123, 605–611.
Subramaniam, J. R., Lyons, W. E., Liu, J., Bartnikas, T. B., Rothstein, J., Price, D. L., et al. (2002). Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nature Neuroscience, 5, 301–307.
Takeda, A., Hirate, M., Tamano, H., & Oku, N. (2003). Release of glutamate and GABA in the hippocampus under zinc deficiency. Journal of Neuroscience Research, 72, 537–542.
Tarohda, T., Yamamoto, M., & Amamo, R. (2004). Regional distribution of manganese, iron, copper, and zinc in the rat brain during development. Analytical and Bioanalytical Chemistry, 380, 240–246.
Thackray, A. M., Knight, R., Haswell, S. J., Bujdoso, R., & Brown, D. R. (2002). Metal imbalance and compromised antioxidant function are early changes in prion disease. Biochemical Journal, 362, 253–258.
Tiwari, A., Liba, A., Sohn, S. H., Seetharaman, S. V., Bilsel, O., Matthews, C. R., et al. (2009). Metal deficiency increases aberrant hydrophobicity of mutant superoxide dismutases that cause amyotrophic lateral sclerosis. Journal of Biological Chemistry (in press).
Tsenkova, R. N., Iordanova, I. K., Toyoda, K., & Brown, D. R. (2004). Prion protein fate governed by metal binding. Biochemical and Biophysical Research Communications, 325, 1005–1012.
Uversky, V. N., Li, J., & Fink, A. L. (2001). Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. Journal of Biological Chemistry, 276, 44284–44296.
Varela-Nallar, L., Toledo, E. M., Larrondo, F. L., Cabral, A. L., Martins, V. R., & Inestrosa, N. C. (2006). Induction of cellular prion protein gene expression by copper in neurons. American Journal of Physiology and Cell Physiology, 290, 271–281.
Vašák, M., & Meloni, G. (2008). Metallothionein structure and reactivity. In: Metallothioneins in biochemistry and pathology (pp. 3–27). Singapore, London: World Scientific.
Wadsworth, J. D. F., & Collinge, J. (2007). Update on human prion disease. Biochemical and Biophysical Acta, 1772, 598–609.
Waggoner, D. J., Drisaldi, B., Bartnikas, T. B., Casareno, R. L., Prohaska, J. R., Gitlin, J. D., et al. (2000). Brain copper content and cuproenzyme activity do not vary with prion protein expression. Journal of Biological Chemistry, 275, 7455–7458.
Weiss, J. H., Sensi, S. L., & Koh, J. Y. (2000). Zn(2+): A novel ionic mediator of neural injury in brain disease. Trends in Pharmacological Sciences, 21, 395–401.
Wells, M. A., Jackson, G. S., Jones, S., Hosszu, L. L., Craven, C. J., Clarke, A. R., et al. (2006). A reassessment of copper(II) binding in the full length prion protein. Biochemical Journal, 399, 435–444.
White, A. R., Du, T., Laughton, K. M., Volitakis, I., Sharples, R. A., Xilinas, M. E., et al. (2006). Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. Journal of Biological Chemistry, 281, 17670–17680.
Wong, B. S., Brown, D. R., Pan, T., Whiteman, M., Liu, T., Bu, X., et al. (2001). Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. Journal of Neurochemistry, 79, 689–698.
Yim, M. B., Chock, P. B., & Stadtman, E. R. (2003). Copper-zinc superoxide dismutase and familial amyotrophic lateral sclerosis. In Metal ions and neurodegenerative disorders (pp. 263–278). Singapore, London: World Scientific.
Yokel, R. A. (1994). Aluminum chelation: Chemistry, clinical, and experimental studies and the search for alternatives to desferrioxamine. Journal of Toxicology and Environmental Health, 41, 131–174.
Yumoto, S., Kakimi, S., Ohsaki, A., & Ishikawa, A. (2009). Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer’s disease. Journal of Inorganic Biochemistry (in press).
Zambenedetti, P., Giordano, R., & Zatta, P. (1998). Metallothioneins are highly expressed in astrocytes and microcapillaries in Alzheimer’s disease. Journal of Chemical Neuroanatomy, 15, 21–26.
Zatta, P. (Ed.). (2002). Recent topics in aluminium chemistry. Coordination Chemistry Reviews, 228, 91–396.
Zatta, P. (Ed.). (2003). Metal ions and neurodegenerative disorders (pp. 1–508). Singapore, London: World Scientific.
Zatta, P. (2006). Aluminum and Alzheimer’s disease: A Vexata Questio between uncertain data and a lot of imagination. Journal of Alzheimers Diseases, 10, 33–37.
Zatta, P. (Ed.). (2008). Metallothioneins in biochemistry and pathology (pp. 1–320). Singapore, London: World Scientific.
Zatta, P., Drago, D., Bolognin, S., & Sensi, S. L. (2009). Alzheimer’s disease, metal ions and metabolic homeostatic therapy. Trends in Pharmacological Sciences, 30, 346–355.
Zatta, P., Drago, D., Zambenedetti, P., Bolognin, S., Nogara, E., Peruffo, A., et al. (2008). Accumulation of copper and other metal ions, and metallothionein I/II expression in the bovine brain as a function of aging. Journal of Chemical Neuroanatomy, 36, 1–5.
Zatta, P., & Frank, A. (2007). Copper deficiency and neurological disorders in man and animals. Brain Research Reviews, 54, 19–33.
Zatta, P., Zambenedetti, P., Reusche, E., Stellmacher, F., Cester, A., Albanese, P., et al. (2004). A fatal case of aluminium encephalopathy in a patient with severe chronic renal failure not on dialysis. Nephrology, Dialysis, Transplantation, 19, 2929–2931.
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R., & Crichton, R. R. (2004). Iron, brain ageing and neurodegenerative disorders. Nature Reviews Neuroscience, 5, 863–873.
Zhou, C., Huang, Y., & Przedborski, S. (2008). Oxidative stress in Parkinson’s disease: A mechanism of pathogenic and therapeutic significance. Annals of the New York Academy of Sciences, 1147, 93–104.
Acknowledgments
This work was supported by Grants from CNR/MIUR (FIRB No. RBNE03PX83) and PRIN 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolognin, S., Messori, L. & Zatta, P. Metal Ion Physiopathology in Neurodegenerative Disorders. Neuromol Med 11, 223–238 (2009). https://doi.org/10.1007/s12017-009-8102-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-009-8102-1