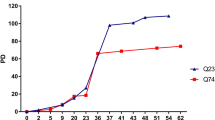
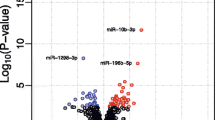
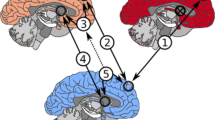
Abstract
Huntington’s disease (HD) is an incurable, fatal neurodegenerative disorder that is caused by a polyglutamine expansion in the huntingtin (Htt) protein. Neuronal death in the striatum—the most obvious manifestation of the disease—is likely to result from widespread dysregulation of gene expression in various brain regions. To date, several potential mechanisms for this have been discovered, including one involving REST (RE1-Silencing Transcription Factor), a master regulator of neuronal genes. Recently, independent studies have demonstrated that post-transcriptional gene regulation by microRNAs is also disrupted in HD. Expression of key neuronal microRNAs—including mir-9/9*, mir-124 and mir-132—is repressed in the brains of human HD patients and mouse models. These changes occur downstream of REST, and are likely to result in major disruption of mRNA regulation and neuronal function. In this study we will discuss these findings and their implications for our understanding of HD. Using updated bioinformatic analysis, we predict 21 new candidate microRNAs in HD. We propose future strategies for unifying large-scale transcriptional and microRNA datasets with the aim of explaining HD aetiology. By way of example, we show how available genomic datasets can be integrated to provide independent, analytical validation for dysregulation of REST and microRNA mir-124 in HD. As a consequence, gene ontology analysis indicates that HD is characterised by a broad-based depression of neural genes in the caudate and motor cortex. Thus, we propose that a combination of REST, microRNAs and possibly other non-coding RNAs profoundly affect the neuronal transcriptome in HD.



Similar content being viewed by others
Abbreviations
- HD:
-
Huntington’s disease
- Htt:
-
Huntingtin
- mutHtt:
-
Mutant huntingtin
- BDNF:
-
Brain-derived neurotrophic factor
- miRNA:
-
MicroRNA
- REST:
-
Repressor element 1-silencing transcription factor
- RE1:
-
Repressor element 1
- NRSF:
-
Neuron-restrictive silencing factor
- NRSE:
-
Neuron-restrictive silencing element
- SCA:
-
Spinocerebellar ataxia
- SP1:
-
Specificity protein 1
- RISC:
-
RNA-induced silencing complex
- DRPLA:
-
Dentatorubral-pallidoluysian atrophy
- SBMA:
-
Spinobulbar muscular atrophy
- MRE:
-
MicroRNA response element
- CREB:
-
cAMP response element binding
- MeCP2:
-
Methyl CpG binding protein 2
- BACE1:
-
β-site of APP cleaving enzyme
- AD:
-
Alzheimer’s disease
- ncRNA:
-
Noncoding RNA
- ChIP-Seq:
-
Chromatin immunoprecipitation coupled to high-throughput sequencing
References
Abelson, J. F., Kwan, K. Y., O’Roak, B. J., Baek, D. Y., Stillman, A. A., et al. (2005). Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science, 310, 317–320. doi:10.1126/science.1116502.
Aboobaker, A. A., Tomancak, P., Patel, N., Rubin, G. M., & Lai, E. C. (2005). Drosophila microRNAs exhibit diverse spatial expression patterns during embryonic development. Proceedings of the National Academy of Sciences of the United States of America, 102, 18017–18022. doi:10.1073/pnas.0508823102.
Altar, C. A., Cai, N., Bliven, T., Juhasz, M., Conner, J. M., et al. (1997). Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature, 389, 856–860. doi:10.1038/39885.
Arzberger, T., Krampfl, K., Leimgruber, S., & Weindl, A. (1997). Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington’s disease—an in situ hybridization study. Journal of Neuropathology and Experimental Neurology, 56, 440–454. doi:10.1097/00005072-199704000-00013.
Augood, S. J., Faull, R. L., & Emson, P. C. (1997). Dopamine D1 and D2 receptor gene expression in the striatum in Huntington’s disease. Annals of Neurology, 42, 215–221. doi:10.1002/ana.410420213.
Bae, B.-I., Xu, H., Igarashi, S., Fujimuro, M., Agrawal, N., et al. (2005). p53 mediates cellular dysfunction and behavioral abnormalities in huntington’s disease. Neuron, 47, 29–41. doi:10.1016/j.neuron.2005.06.005.
Bak, M., Silahtaroglu, A., Moller, M., Christensen, M., Rath, M. F., et al. (2008). MicroRNA expression in the adult mouse central nervous system. RNA, 14, 432–444. doi:10.1261/rna.783108.
Ballas, N., Battaglioli, E., Atouf, F., Andres, M. E., Chenoweth, J., et al. (2001). Regulation of neuronal traits by a novel transcriptional complex. Neuron, 31, 353–365. doi:10.1016/S0896-6273(01)00371-3.
Bartel, D. P. (2003). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116, 281–297. doi:10.1016/S0092-8674(04)00045-5.
Bartel, D. P. (2009). MicroRNAs: Target recognition and regulatory functions. Cell, 136, 215–233. doi:10.1016/j.cell.2009.01.002.
Benn, C. L., Sun, T., Sadri-Vakili, G., McFarland, K. N., DiRocco, D. P., et al. (2008). Huntingtin modulates transcription, occupies gene promoters in vivo, and binds directly to DNA in a polyglutamine-dependent manner. Journal of Neuroscience, 28, 10720–10733. doi:10.1523/JNEUROSCI.2126-08.2008.
Bilen, J., Liu, N., Burnett, B., Pittman, R., & Bonini, N. (2006). MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Molecular Cell, 24, 157–163. doi:10.1016/j.molcel.2006.07.030.
Borovecki, F., Lovrecic, L., Zhou, J., Jeong, H., Then, F., et al. (2005). Genome-wide expression profiling of human blood reveals biomarkers for Huntington’s disease. PNAS, 102, 11023–11028. doi:10.1073/pnas.0504921102.
Boutell, J. M., Thomas, P., Neal, J. W., Weston, V. J., Duce, J., et al. (1999). Aberrant interactions of transcriptional repressor proteins with the Huntington’s disease gene product, huntingtin. Human Molecular Genetics, 8, 1647–1655. doi:10.1093/hmg/8.9.1647.
Bruce, A. W., Donaldson, I. J., Wood, I. C., Yerbury, S. A., Sadowski, M. I., et al. (2004). Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. PNAS, 101, 10458–10463. doi:10.1073/pnas.0401827101.
Calderone, A., Jover, T., Noh, K.-M., Tanaka, H., Yokota, H., et al. (2003). Ischemic insults derepress the gene silencer REST in neurons destined to die. Journal of Neuroscience, 23, 2112–2121.
Care, A., Catalucci, D., Felicetti, F., Bonci, D., Addario, A., et al. (2007). MicroRNA-133 controls cardiac hypertrophy. Nature Medicine, 13, 613–618. doi:10.1038/nm1582.
Carninci, P., Kasukawa, T., Katayama, S., Gough, J., Frith, M. C., et al. (2005). The transcriptional landscape of the mammalian genome. Science, 309, 1559–1563. doi:10.1126/science.1112014.
Cattaneo, E., Zuccato, C., & Tartari, M. (2005). Normal huntingtin function: an alternative approach to Huntington’s disease. Nature Reviews Neuroscience, 6, 919–930. doi:10.1038/nrn1806.
Cha, J. H. (2007). Transcriptional signatures in Huntington’s disease. Progress in Neurobiology, 83, 228–248. doi:10.1016/j.pneurobio.2007.03.004.
Chen, J.-F., Mandel, E. M., Thomson, J. M., Wu, Q., Callis, T. E., et al. (2006). The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nature Genetics, 38, 228–233. doi:10.1038/ng1725.
Chen, Z.-F., Paquette, A. J., & Anderson, D. J. (1998). NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nature Genetics, 20, 136–142. doi:10.1038/2431.
Chen-Plotkin, A. S., Sadri-Vakili, G., Yohrling, G. J., Braveman, M. W., Benn, C. L., et al. (2006). Decreased association of the transcription factor Sp1 with genes downregulated in Huntington’s disease. Neurobiology of Disease, 22, 233–241. doi:10.1016/j.nbd.2005.11.001.
Chong, J., Tapia-Ramirez, J., Kim, S., Toledo-Aral, J., Zheng, Y., et al. (1995). REST: A mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell, 80, 949–957. doi:10.1016/0092-8674(95)90298-8.
Clemson, C. M., Hutchinson, J. N., Sara, S. A., Ensminger, A. W., Fox, A. H., et al. (2009). An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Molecular Cell, 33(6), 717–726.
Conaco, C., Otto, S., Han, J.-J., & Mandel, G. (2006). Reciprocal actions of REST and a microRNA promote neuronal identity. PNAS, 103, 2422–2427. doi:10.1073/pnas.0511041103.
Dinger, M. E., Amaral, P. P., Mercer, T. R., Pang, K. C., Bruce, S. J., et al. (2008). Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Research, 18, 1433–1445. doi:10.1101/gr.078378.108.
Dunah, A. W., Jeong, H., Griffin, A., Kim, Y. M., Standaert, D. G., et al. (2002). Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science, 296, 2238–2243. doi:10.1126/science.1072613.
Eskenazi, B. R., Wilson-Rich, N. S., & Starks, P. T. (2007). A Darwinian approach to Huntington’s disease: Subtle health benefits of a neurological disorder. Medical Hypotheses, 69, 1183–1189. doi:10.1016/j.mehy.2007.02.046.
Faghihi, M. A., Modarresi, F., Khalil, A. M., Wood, D. E., Sahagan, B. G., et al. (2008). Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nature Medicine, 14, 723–730. doi:10.1038/nm1784.
Farh, K. K., Grimson, A., Jan, C., Lewis, B. P., Johnston, W. K., et al. (2005). The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science, 310, 1817–1821. doi:10.1126/science.1121158.
Feng, J., Bi, C., Clark, B. S., Mady, R., Shah, P., et al. (2006). The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes and Development, 20, 1470–1484. doi:10.1101/gad.1416106.
Ferrante, R. J., Kowall, N. W., Beal, M. F., Richardson, E. P., Jr., Bird, E. D., et al. (1985). Selective sparing of a class of striatal neurons in Huntington’s disease. Science, 230, 561–563. doi:10.1126/science.2931802.
Filipowicz, W., Bhattacharyya, S. N., & Sonenberg, N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nature Reviews. Genetics, 9, 102–114. doi:10.1038/nrg2290.
Friedlander, M. R., Chen, W., Adamidi, C., Maaskola, J., Einspanier, R., et al. (2008). Discovering microRNAs from deep sequencing data using miRDeep. Nature Biotechnology, 26, 407–415. doi:10.1038/nbt1394.
Gangaraju, V. K., & Lin, H. (2009). MicroRNAs: Key regulators of stem cells. Nature Reviews. Molecular Cell Biology, 10, 116–125. doi:10.1038/nrm2621.
Giraldez, A. J., Cinalli, R. M., Glasner, M. E., Enright, A. J., Thomson, J. M., et al. (2005). MicroRNAs regulate brain morphogenesis in zebrafish. Science, 308, 833–838. doi:10.1126/science.1109020.
Greenway, D. J., Street, M., Jeffries, A., & Buckley, N. J. (2007). RE1 silencing transcription factor maintains a repressive chromatin environment in embryonic hippocampal neural stem cells. Stem Cells, 25, 354–363. doi:10.1634/stemcells.2006-0207.
Griffiths-Jones, S. (2004). The microRNA registry. Nucleic Acids Research, 32, D109–D111. doi:10.1093/nar/gkh023.
He, L., He, X., Lim, L. P., de Stanchina, E., Xuan, Z., et al. (2007). A microRNA component of the p53 tumour suppressor network. Nature, 447, 1130–1134. doi:10.1038/nature05939.
He, L., Thomson, J. M., Hemann, M. T., Hernando-Monge, E., Mu, D., et al. (2005). A microRNA polycistron as a potential human oncogene. Nature, 435, 828–833. doi:10.1038/nature03552.
Hebert, S. S., Horre, K., Nicolai, L., Papadopoulou, A. S., Mandemakers, W., et al. (2008). Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America, 105, 6415–6420. doi:10.1073/pnas.0710263105.
Hedreen, J. C., Peyser, C. E., Folstein, S. E., & Ross, C. A. (1991). Neuronal loss in layers V and VI of cerebral cortex in Huntington’s disease. Neuroscience Letters, 133, 257–261. doi:10.1016/0304-3940(91)90583-F.
Hodges, A., Strand, A. D., Aragaki, A. K., Kuhn, A., Sengstag, T., et al. (2006). Regional and cellular gene expression changes in human Huntington’s disease brain. Human Molecular Genetics, 15, 965–977. doi:10.1093/hmg/ddl013.
Huang, C. C., Faber, P. W., Persichetti, F., Mittal, V., Vonsattel, J. P., et al. (1998). Amyloid formation by mutant huntingtin: Threshold, progressivity and recruitment of normal polyglutamine proteins. Somatic Cell and Molecular Genetics, 24, 217–233. doi:10.1023/B:SCAM.0000007124.19463.e5.
Huntington’s Disease Collaborative Research Group. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell, 72, 971–983. doi:10.1016/0092-8674(93)90585-E.
Ji, P., Diederichs, S., Wang, W., Boing, S., Metzger, R., et al. (2003). MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene, 22, 8031–8041. doi:10.1038/sj.onc.1206928.
Johnson, R., Gamblin, R. J., Ooi, L., Bruce, A. W., Donaldson, I. J., et al. (2006). Identification of the REST regulon reveals extensive transposable element-mediated binding site duplication. Nucleic Acids Research, 34, 3862–3877. doi:10.1093/nar/gkl525.
Johnson, D., Mortazavi, A., Myers, R., & Wold, B. (2007). Genome-wide mapping of in vivo protein-DNA interactions. Science, 316, 1497–1502. doi:10.1126/science.1141319.
Johnson, R., Teh, C. H., Jia, H., Vanisri, R. R., Pandey, T., et al. (2009). Regulation of neural macroRNAs by the transcriptional repressor REST. RNA, 15, 85–96. doi:10.1261/rna.1127009.
Johnson, R., Teh, C. H.-L., Kunarso, G., Wong, K. Y., Srinivasan, G., et al. (2008a). REST regulates distinct transcriptional networks in embryonic and neural stem cells. PLoS Biology, 6, e256. doi:10.1371/journal.pbio.0060256.
Johnson, R., Zuccato, C., Belyaev, N. D., Guest, D. J., Cattaneo, E., et al. (2008b). A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiology of Disease, 29, 438–445. doi:10.1016/j.nbd.2007.11.001.
Karres, J. S., Hilgers, V., Carrera, I., Treisman, J., & Cohen, S. M. (2007). The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell, 131, 136–145. doi:10.1016/j.cell.2007.09.020.
Kegel, K. B., Meloni, A. R., Yi, Y., Kim, Y. J., Doyle, E., et al. (2002). Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription. Journal of Biological Chemistry, 277, 7466–7476. doi:10.1074/jbc.M103946200.
Kim, M. O., Chawla, P., Overland, R. P., Xia, E., Sadri-Vakili, G., et al. (2008). Altered histone monoubiquitylation mediated by mutant huntingtin induces transcriptional dysregulation. Journal of Neuroscience, 28, 3947–3957. doi:10.1523/JNEUROSCI.5667-07.2008.
Kim, J., Inoue, K., Ishii, J., Vanti, W. B., Voronov, S. V., et al. (2007). A MicroRNA feedback circuit in midbrain dopamine neurons. Science, 317, 1220–1224. doi:10.1126/science.1140481.
Kosik, K. S. (2006). The neuronal microRNA system. Nature Reviews Neuroscience, 7, 911–920. doi:10.1038/nrn2037.
Krichevsky, A. M., King, K. S., Donahue, C. P., Khrapko, K., & Kosik, K. S. (2003). A microRNA array reveals extensive regulation of microRNAs during brain development. RNA, 9, 1274–1281. doi:10.1261/rna.5980303.
Krichevsky, A. M., Sonntag, K. C., Isacson, O., & Kosik, K. S. (2006). Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells, 24, 857–864. doi:10.1634/stemcells.2005-0441.
Kuhn, A., Goldstein, D. R., Hodges, A., Strand, A. D., Sengstag, T., et al. (2007). Mutant huntingtin’s effects on striatal gene expression in mice recapitulate changes observed in human Huntington’s disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Human Molecular Genetics, 16, 1845–1861. doi:10.1093/hmg/ddm133.
Landgraf, P., Rusu, M., Sheridan, R., Sewer, A., Iovino, N., et al. (2007). A mammalian microRNA expression atlas based on small RNA library sequencing. Cell, 129, 1401–1414. doi:10.1016/j.cell.2007.04.040.
Lanz, R., McKenna, N., Onate, S., Albrecht, U., Wong, J., et al. (1999). A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell, 97, 17–27. doi:10.1016/S0092-8674(00)80711-4.
Leone, S., Mutti, C., Kazantsev, A., Sturlese, M., Moro, S., et al. (2008). SAR and QSAR study on 2-aminothiazole derivatives, modulators of transcriptional repression in Huntington’s disease. Bioorganic & Medicinal Chemistry, 16, 5695–5703. doi:10.1016/j.bmc.2008.03.067.
Leucht, C., Stigloher, C., Wizenmann, A., Klafke, R., Folchert, A., et al. (2008). MicroRNA-9 directs late organizer activity of the midbrain–hindbrain boundary. Nature Neuroscience, 11, 641–648. doi:10.1038/nn.2115.
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P., & Burge, C. B. (2003). Prediction of mammalian microRNA targets. Cell, 115, 787–798. doi:10.1016/S0092-8674(03)01018-3.
Lim, L. P., Lau, N. C., Garrett-Engele, P., Grimson, A., Schelter, J. M., et al. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature, 433, 769–773. doi:10.1038/nature03315.
Lipovich, L., Vanisri, R. R., Kong, S. L., Lin, C. Y., & Liu, E. T. (2006). Primate-specific endogenous cis-antisense transcription in the human 5q31 protocadherin gene cluster. Journal of Molecular Evolution, 62, 73–88. doi:10.1007/s00239-005-0041-3.
Louro, R., Nakaya, H. I., Amaral, P. P., Festa, F., Sogayar, M. C., et al. (2007). Androgen responsive intronic non-coding RNAs. BMC Biology, 5, 4. doi:10.1186/1741-7007-5-4.
Lu, J., Getz, G., Miska, E. A., Alvarez-Saavedra, E., Lamb, J., et al. (2005). MicroRNA expression profiles classify human cancers. Nature, 435, 834–838. doi:10.1038/nature03702.
Luthi-Carter, R., Hanson, S. A., Strand, A. D., Bergstrom, D. A., Chun, W., et al. (2002). Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Human Molecular Genetics, 11, 1911–1926. doi:10.1093/hmg/11.17.1911.
Luthi-Carter, R., Strand, A., Peters, N. L., Solano, S. M., Hollingsworth, Z. R., et al. (2000). Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Human Molecular Genetics, 9, 1259–1271. doi:10.1093/hmg/9.9.1259.
Makeyev, E. V., Zhang, J., Carrasco, M. A., & Maniatis, T. (2007). The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular Cell, 27, 435–448. doi:10.1016/j.molcel.2007.07.015.
Mangiarini, L., Sathasivam, K., Seller, M., Cozens, B., Harper, A., et al. (1996). Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell, 87, 493–506. doi:10.1016/S0092-8674(00)81369-0.
Mariner, P. D., Walters, R. D., Espinoza, C. A., Drullinger, L. F., Wagner, S. D., et al. (2008). Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Molecular Cell, 29, 499–509. doi:10.1016/j.molcel.2007.12.013.
McCampbell, A., Taylor, J. P., Taye, A. A., Robitschek, J., Li, M., et al. (2000). CREB-binding protein sequestration by expanded polyglutamine. Human Molecular Genetics, 9, 2197–2202. doi:10.1093/hmg/9.14.2197.
Mehler, M. F., & Mattick, J. S. (2007). Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiological Reviews, 87, 799–823. doi:10.1152/physrev.00036.2006.
Meister, G., Landthaler, M., Peters, L., Chen, P. Y., Urlaub, H., et al. (2005). Identification of novel argonaute-associated proteins. Current Biology, 15, 2149–2155. doi:10.1016/j.cub.2005.10.048.
Mercer, T. R., Dinger, M. E., Sunkin, S. M., Mehler, M. F., & Mattick, J. S. (2008). Specific expression of long noncoding RNAs in the mouse brain. Proceedings of the National Academy of Sciences of the United States of America, 105, 716–721. doi:10.1073/pnas.0706729105.
Miska, E. A., Alvarez-Saavedra, E., Townsend, M., Yoshii, A., Sestan, N., et al. (2004). Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biology, 5, R68. doi:10.1186/gb-2004-5-9-r68.
Mortazavi, A., Thompson, E. C. L., Garcia, S. T., Myers, R. M., & Wold, B. (2006). Comparative genomics modeling of the NRSF/REST repressor network: From single conserved sites to genome-wide repertoire. Genome Research, 16, 1208–1221. doi:10.1101/gr.4997306.
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L., & Wold, B. (2008). Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods, 5, 621–628. doi:10.1038/nmeth.1226.
Nakamura, K., Jeong, S. Y., Uchihara, T., Anno, M., Nagashima, K., et al. (2001). SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Human Molecular Genetics, 10, 1441–1448. doi:10.1093/hmg/10.14.1441.
Nomura, T., Kimura, M., Horii, T., Morita, S., Soejima, H., et al. (2008). MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Human Molecular Genetics, 17, 1192–1199. doi:10.1093/hmg/ddn011.
Olson, M. V., & Varki, A. (2003). Sequencing the chimpanzee genome: insights into human evolution and disease. Nature Review. Genetics, 4, 20–28. doi:10.1038/nrg981.
Ooi, L., & Wood, I. C. (2007). Chromatin crosstalk in development and disease: lessons from REST. Nature Reviews Genetics, 8, 544–554. doi:10.1038/nrg2100.
Otto, S. J., McCorkle, S. R., Hover, J., Conaco, C., Han, J.-J., et al. (2007). A new binding motif for the transcriptional repressor rest uncovers large gene networks devoted to neuronal functions. Journal of Neuroscience, 27, 6729–6739. doi:10.1523/JNEUROSCI.0091-07.2007.
Packer, A. N., Xing, Y., Harper, S. Q., Jones, L., & Davidson, B. L. (2008). The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. Journal of Neuroscience, 28, 14341–14346. doi:10.1523/JNEUROSCI.2390-08.2008.
Palm, K., Belluardo, N., Metsis, M., & To, Timmusk. (1998). Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. Journal of Neuroscience, 18, 1280–1296.
Pandey, R. R., Mondal, T., Mohammad, F., Enroth, S., Redrup, L., et al. (2008). Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Molecular Cell, 32, 232–246. doi:10.1016/j.molcel.2008.08.022.
Patel, N., Hoang, D., Miller, N., Ansaloni, S., Huang, Q., et al. (2008). MicroRNAs can regulate human APP levels. Molecular Neurodegeneration, 3, 10. doi:10.1186/1750-1326-3-10.
Perez, D. S., Hoage, T. R., Pritchett, J. R., Ducharme-Smith, A. L., Halling, M. L., et al. (2008). Long, abundantly expressed non-coding transcripts are altered in cancer. Human Molecular Genetics, 17, 642–655. doi:10.1093/hmg/ddm336.
Pollard, K. S., Salama, S. R., Lambert, N., Lambot, M.-A., Coppens, S., et al. (2006). An RNA gene expressed during cortical development evolved rapidly in humans. Nature, 443, 167–172. doi:10.1038/nature05113.
Ponjavic, J., Ponting, C. P., & Lunter, G. (2007). Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Research, 17, 556–565. doi:10.1101/gr.6036807.
Preker, P., Nielsen, J., Kammler, S., Lykke-Andersen, S., Christensen, M. S., et al. (2008). RNA exosome depletion reveals transcription upstream of active human promoters. Science, 322, 1851–1854. doi:10.1126/science.1164096.
Qiu, Z., Norflus, F., Singh, B., Swindell, M. K., Buzescu, R., et al. (2006). Sp1 is up-regulated in cellular and transgenic models of Huntington disease, and its reduction is neuroprotective. Journal of Biological Chemistry, 281, 16672–16680. doi:10.1074/jbc.M511648200.
Rajewsky, N., & Socci, N. D. (2004). Computational identification of microRNA targets. Developmental Biology, 267, 529–535. doi:10.1016/j.ydbio.2003.12.003.
Ravasi, T., Suzuki, H., Pang, K. C., Katayama, S., Furuno, M., et al. (2006). Experimental validation of the regulated expression of large numbers of non-coding RNAs from the mouse genome. Genome Research, 16, 11–19. doi:10.1101/gr.4200206.
Reiner, A., Albin, R. L., Anderson, K. D., D’Amato, C. J., Penney, J. B., et al. (1988). Differential loss of striatal projection neurons in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America, 85, 5733–5737. doi:10.1073/pnas.85.15.5733.
Rigamonti, D., Bolognini, D., Mutti, C., Zuccato, C., Tartari, M., et al. (2007). Loss of huntingtin function complemented by small molecules acting as repressor element 1/neuron restrictive silencer element silencer modulators. Journal of Biological Chemistry, 282, 24554–24562. doi:10.1074/jbc.M609885200.
Rinn, J., Kertesz, M., Wang, J., Squazzo, S., Xu, X., et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell, 127, 1311–1323. doi:10.1016/j.cell.2007.05.022.
Runne, H., Kuhn, A., Wild, E. J., Pratyaksha, W., Kristiansen, M., et al. (2007). Analysis of potential transcriptomic biomarkers for Huntington’s disease in peripheral blood. Proceedings of the National Academy of Sciences of the United States of America, 104, 14424–14429. doi:10.1073/pnas.0703652104.
Ruvkun, G. (2001). Molecular biology. Glimpses of a tiny RNA world. Science, 294, 797–799. doi:10.1126/science.1066315.
Saba, R., Goodman, C. D., Huzarewich, R. L., Robertson, C., & Booth, S. A. (2008). A miRNA signature of prion induced neurodegeneration. PLoS ONE, 3, e3652. doi:10.1371/journal.pone.0003652.
Sadri-Vakili, G., Bouzou, B., Benn, C. L., Kim, M. O., Chawla, P., et al. (2007). Histones associated with downregulated genes are hypo-acetylated in Huntington’s disease models. Human Molecular Genetics, 16, 1293–1306. doi:10.1093/hmg/ddm078.
Savas, J. N., Makusky, A., Ottosen, S., Baillat, D., Then, F., et al. (2008). Huntington’s disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. Proceedings of the National Academy of Sciences of the United States of America, 105, 10820–10825. doi:10.1073/pnas.0800658105.
Schaefer, A., O’Carroll, D., Tan, C. L., Hillman, D., Sugimori, M., et al. (2007). Cerebellar neurodegeneration in the absence of microRNAs. Journal of Experimental Medicine, 204, 1553–1558. doi:10.1084/jem.20070823.
Schoenherr, C., & Anderson, D. (1995). The neuron-restrictive silencer factor (NRSF): A coordinate repressor of multiple neuron-specific genes. Science, 5202, 1360–1363. doi:10.1126/science.7871435.
Schoenherr, C. J., Paquette, A. J., & Anderson, D. J. (1996). Identification of potential target genes for the neuron-restrictive silencer factor. Proceedings of the National Academy of Sciences, 93, 9881–9886. doi:10.1073/pnas.93.18.9881.
Schratt, G. M., Tuebing, F., Nigh, E. A., Kane, C. G., Sabatini, M. E., et al. (2006). A brain-specific microRNA regulates dendritic spine development. Nature, 439, 283–289. doi:10.1038/nature04367.
Sempere, L. F., Freemantle, S., Pitha-Rowe, I., Moss, E., Dmitrovsky, E., et al. (2004). Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biology, 5, R13. doi:10.1186/gb-2004-5-3-r13.
Shibata, M., Kurokawa, D., Nakao, H., Ohmura, T., & Aizawa, S. (2008). MicroRNA-9 modulates Cajal–Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. Journal of Neuroscience, 28, 10415–10421. doi:10.1523/JNEUROSCI.3219-08.2008.
Shimojo, M. (2008). Huntingtin regulates RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) nuclear trafficking indirectly through a complex with REST/NRSF-interacting LIM domain protein (RILP) and dynactin p150 Glued. Journal of Biological Chemistry, 283, 34880–34886. doi:10.1074/jbc.M804183200.
Smirnova, L., Grafe, A., Seiler, A., Schumacher, S., Nitsch, R., et al. (2005). Regulation of miRNA expression during neural cell specification. European Journal of Neuroscience, 21, 1469–1477. doi:10.1111/j.1460-9568.2005.03978.x.
Snell, R. G., MacMillan, J. C., Cheadle, J. P., Fenton, I., Lazarou, L. P., et al. (1993). Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington’s disease. Nature Genetics, 4, 393–397. doi:10.1038/ng0893-393.
Sood, P., Krek, A., Zavolan, M., Macino, G., & Rajewsky, N. (2006). Cell-type-specific signatures of microRNAs on target mRNA expression. PNAS, 103, 2746–2751. doi:10.1073/pnas.0511045103.
Stark, K. L., Xu, B., Bagchi, A., Lai, W. S., Liu, H., et al. (2008). Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nature Genetics, 40, 751–760. doi:10.1038/ng.138.
Steffan, J. S., Kazantsev, A., Spasic-Boskovic, O., Greenwald, M., Zhu, Y.-Z., et al. (2000). The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. PNAS, 97, 6763–6768. doi:10.1073/pnas.100110097.
Strand, A. D., Baquet, Z. C., Aragaki, A. K., Holmans, P., Yang, L., et al. (2007). Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. Journal of Neuroscience, 27, 11758–11768. doi:10.1523/JNEUROSCI.2461-07.2007.
Sun, Y.-M., Greenway, D. J., Johnson, R., Street, M., Belyaev, N. D., et al. (2005). Distinct profiles of REST interactions with its target genes at different stages of neuronal development. Molecular Biology of the Cell, 16, 5630–5638. doi:10.1091/mbc.E05-07-0687.
Tochitani, S., & Hayashizaki, Y. (2008). Nkx2.2 antisense RNA overexpression enhanced oligodendrocytic differentiation. Biochemical and Biophysical Research Communications, 372, 691–696. doi:10.1016/j.bbrc.2008.05.127.
Varki, A., & Altheide, T. K. (2005). Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Research, 15, 1746–1758. doi:10.1101/gr.3737405.
Visvanathan, J., Lee, S., Lee, B., Lee, J. W., & Lee, S. K. (2007). The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes and Development, 21, 744–749. doi:10.1101/gad.1519107.
Vo, N., Klein, M. E., Varlamova, O., Keller, D. M., Yamamoto, T., et al. (2005). From the cover: A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. PNAS, 102, 16426–16431. doi:10.1073/pnas.0508448102.
Vonsattel, J. P., & DiFiglia, M. (1998). Huntington disease. Journal of Neuropathology and Experimental Neurology, 57, 369–384. doi:10.1097/00005072-199805000-00001.
Walker, F. O. (2007). Huntington’s disease. Lancet, 369, 218–228. doi:10.1016/S0140-6736(07)60111-1.
Wang, W. X., Rajeev, B. W., Stromberg, A. J., Ren, N., Tang, G., et al. (2008). The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. Journal of Neuroscience, 28, 1213–1223. doi:10.1523/JNEUROSCI.5065-07.2008.
Wayman, G. A., Davare, M., Ando, H., Fortin, D., Varlamova, O., et al. (2008). An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proceedings of the National Academy of Sciences of the United States of America, 105, 9093–9098. doi:10.1073/pnas.0803072105.
Wheeler, V. C., Auerbach, W., White, J. K., Srinidhi, J., Auerbach, A., et al. (1999). Length-dependent gametic CAG repeat instability in the Huntington’s disease knock-in mouse. Human Molecular Genetics, 8, 115–122. doi:10.1093/hmg/8.1.115.
Willingham, A., Orth, A., Batalov, S., Peters, E., Wen, B., et al. (2005). A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science, 309, 1570–1573. doi:10.1126/science.1115901.
Wu, J., & Xie, X. (2006). Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biology, 7, R85. doi:10.1186/gb-2006-7-9-r85.
Yu, J. Y., Chung, K. H., Deo, M., Thompson, R. C., & Turner, D. L. (2008). MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Experimental Cell Research, 314, 2618–2633. doi:10.1016/j.yexcr.2008.06.002.
Zabel, C., Chamrad, D. C., Priller, J., Woodman, B., Meyer, H. E., et al. (2002). Alterations in the mouse and human proteome caused by Huntington’s disease. Molecular & Cellular Proteomics, 1, 366–375. doi:10.1074/mcp.M200016-MCP200.
Zhao, T., Li, G., Mi, S., Li, S., Hannon, G. J., et al. (2007a). A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes and Development, 21, 1190–1203. doi:10.1101/gad.1543507.
Zhao, Y., Ransom, J. F., Li, A., Vedantham, V., von Drehle, M., et al. (2007b). Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell, 129, 303–317. doi:10.1016/j.cell.2007.03.030.
Zuccato, C., Belyaev, N., Conforti, P., Ooi, L., Tartari, M., et al. (2007). Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in huntington’s disease. Journal of Neuroscience, 27, 6972–6983. doi:10.1523/JNEUROSCI.4278-06.2007.
Zuccato, C., & Cattaneo, E. (2007). Role of brain-derived neurotrophic factor in Huntington’s disease. Progress in Neurobiology, 81, 294–330. doi:10.1016/j.pneurobio.2007.01.003.
Zuccato, C., Ciammola, A., Rigamonti, D., Leavitt, B. R., Goffredo, D., et al. (2001). Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science, 293, 493–498. doi:10.1126/science.1059581.
Zuccato, C., Tartari, M., Crotti, A., Goffredo, D., Valenza, M., et al. (2003). Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nature Genetics, 35, 76–83. doi:10.1038/ng1219.
Acknowledgements
We wish to thank Chiara Zuccato (University of Milan), Andrew M. Thomson (Genome Institute of Singapore) and Lawrence W. Stanton (Genome Institute of Singapore) for advice, discussions and critical reading of the manuscript. RJ is a postdoctoral fellow funded by the Singapore Agency for Science, Technology and Research (A*STAR).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Both the authors are joint corresponding authors.
Rights and permissions
About this article
Cite this article
Johnson, R., Buckley, N.J. Gene Dysregulation in Huntington’s Disease: REST, MicroRNAs and Beyond. Neuromol Med 11, 183–199 (2009). https://doi.org/10.1007/s12017-009-8063-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-009-8063-4