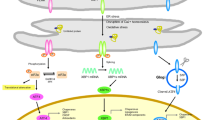
Abstract
Parkinson’s disease (PD) features oxidative stress and accumulation of misfolded (unfolded, alternatively folded, or mutant) proteins with associated loss of dopaminergic neurons. Oxidative stress and the accumulated misfolded proteins elicit cellular responses that include an endoplasmic reticulum (ER) stress response that may protect cells against the toxic buildup of misfolded proteins. Chronic ER stress and accumulation of misfolded proteins in excessive amounts, however, overwhelm the cellular ‘quality control’ system and impair the protective mechanisms designed to promote correct folding and degrade faulty proteins, ultimately leading to organelle dysfunction and neuronal cell death. Paraquat belongs to a class of bipyridyl herbicides and triggers oxidative stress and dopaminergic cell death. Epidemiological studies suggest an increased risk for developing PD following chronic exposure to paraquat. The present study was carried out to determine the role of paraquat in triggering cellular stress particularly ER stress and to elucidate the pathways that couple ER stress to dopaminergic cell death. We demonstrate that paraquat triggers ER stress, cell dysfunction, and dopaminergic cell death. p23, a small co-chaperone protein, is cleaved during ER stress-induced cell death triggered by paraquat and blockage of the caspase cleavage site of p23 was associated with decreased cell death. Paraquat also inhibits proteasomal activity that may further trigger accumulation of misfolded proteins resulting in ER stress. Our results indicate a protective role for p23 in PD-related programmed cell death. The data also underscore the involvement of ER, caspases, and the proteasomal system in ER stress-induced cell death process.






Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- Pcd:
-
Programmed cell death
- eIF2α:
-
Eukaryotic initiation factor-2 alpha
- GRP:
-
Glucose regulated protein
- PD:
-
Parkinson’s disease (PD)
References
Adams, F. S., La Rosa, F. G., Kumar, S., Edwards-Prasad, J., Kentroti, S., Vernadakis, A., et al. (1996). Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochemical Research, 21, 619–627. doi:10.1007/BF02527762.
Andersen, J. K. (2000). What causes the build-up of ubiquitin-containing inclusions in Parkinson’s disease? Mechanisms of Ageing and Development, 118, 15–22. doi:10.1016/S0047-6374(00)00150-0.
Andersen, J. K. (2003). Paraquat and iron exposure as possible synergistic environmental risk factors in Parkinson’s disease. Neurotoxic Research, 5, 307–313.
Andersen, J. K. (2004). Oxidative stress in neurodegeneration: Cause or consequence? Nature Medicine, 10 Suppl, S18–S25. doi:10.1038/nrn1434.
Bose, S., Weikl, T., Bugl, H., & Buchner, J. (1996). Chaperone function of Hsp90-associated proteins. Science, 274, 1715–1717. doi:10.1126/science.274.5293.1715.
Boyce, M., Bryant, K. F., Jousse, C., Long, K., Harding, H. P., Scheuner, D., et al. (2005). A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science, 307, 935–939. doi:10.1126/science.1101902.
Bredesen, D. E., Rao, R. V., & Mehlen, P. (2006). Cell death in the nervous system. Nature, 443, 796–802. doi:10.1038/nature05293.
Bush, K. T., Goldberg, A. L., & Nigam, S. K. (1997). Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. Journal of Biological Chemistry, 272, 9086–9092. doi:10.1074/jbc.272.14.9086.
Caserta, T. M., Smith, A. N., Gultice, A. D., Reedy, M. A., & Brown, T. L. (2003). Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis, 8, 345–352. doi:10.1023/A:1024116916932.
Cheng, E. H., Kirsch, D. G., Clem, R. J., Ravi, R., Kastan, M. B., Bedi, A., et al. (1997). Conversion of Bcl-2 to a Bax-like death effector by caspases. Science, 278, 1966–1968. doi:10.1126/science.278.5345.1966.
Chinta, S. J., & Andersen, J. K. (2005). Dopaminergic neurons. International Journal of Biochemistry and Cell Biology, 37, 942–946. doi:10.1016/j.biocel.2004.09.009.
Chinta, S. J., & Andersen, J. K. (2006). Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: Implications for Parkinson’s disease. Free Radical Biology and Medicine, 41, 1442–1448. doi:10.1016/j.freeradbiomed.2006.08.002.
Chinta, S. J., Kumar, J. M., Zhang, H., Forman, H. J., & Andersen, J. K. (2006). Up-regulation of gamma-glutamyl transpeptidase activity following glutathione depletion has a compensatory rather than an inhibitory effect on mitochondrial complex I activity: Implications for Parkinson’s disease. Free Radical Biology and Medicine, 40, 1557–1563. doi:10.1016/j.freeradbiomed.2005.12.023.
Clarkson, E. D., Rosa, F. G., Edwards-Prasad, J., Weiland, D. A., Witta, S. E., Freed, C. R., et al. (1998). Improvement of neurological deficits in 6-hydroxydopamine-lesioned rats after transplantation with allogeneic simian virus 40 large tumor antigen gene-induced immortalized dopamine cells. Proc Natl Acad Sci USA, 95, 1265–1270. doi:10.1073/pnas.95.3.1265.
Conn, K. J., Gao, W., McKee, A., Lan, M. S., Ullman, M. D., Eisenhauer, P. B., et al. (2004). Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson’s disease and Lewy body pathology. Brain Research, 1022, 164–172. doi:10.1016/j.brainres.2004.07.026.
Conway, K. A., Harper, J. D., & Lansbury, P. T. (1998). Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nature Medicine, 4, 1318–1320. doi:10.1038/3311.
Conway, K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Harper, J. D., Williamson, R. E., et al. (2000). Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Annals of the New York Academy of Sciences, 920, 42–45.
Danielson, S. R., & Andersen, J. K. (2008). Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radical Biology and Medicine, 44, 1789–1794.
Dursun, B., He, Z., Somerset, H., Oh, D. J., Faubel, S., & Edelstein, C. L. (2006). Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. American Journal of Physiology Renal Physiology, 291, F578–F587.
Fei, Q., McCormack, A. L., Di Monte, D. A., & Ethell, D. W. (2008). Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. Journal of Biological Chemistry, 283, 3357–3364. doi:10.1074/jbc.M708451200.
Felts, S. J., & Toft, D. O. (2003). p23, a simple protein with complex activities. Cell Stress Chaperones, 8, 108–113. doi:10.1379/1466-1268(2003)008<0108:PASPWC>2.0.CO;2.
Forman, M. S., Lee, V. M., & Trojanowski, J. Q. (2003). ‘Unfolding’ pathways in neurodegenerative disease. Trends in Neurosciences, 26, 407–410. doi:10.1016/S0166-2236(03)00197-8.
Fribley, A., & Wang, C. Y. (2006). Proteasome inhibitor induces apoptosis through induction of endoplasmic reticulum stress. Cancer Biology & Therapy, 5, 745–748.
Harding, H. P., Calfon, M., Urano, F., Novoa, I., & Ron, D. (2002). Transcriptional and translational control in the Mammalian unfolded protein response. Annual Review of Cell and Developmental Biology, 18, 575–599. doi:10.1146/annurev.cellbio.18.011402.160624.
Hashimoto, M., Rockenstein, E., Crews, L., & Masliah, E. (2003). Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Medicine, 4, 21–36. doi:10.1385/NMM:4:1-2:21.
Holtz, W. A., & O’Malley, K. L. (2003). Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. Journal of Biological Chemistry, 278, 19367–19377. doi:10.1074/jbc.M211821200.
Ishii, Y., Waxman, S., & Germain, D. (2007). Targeting the ubiquitin-proteasome pathway in cancer therapy. Anticancer Agents in Medicinal Chemistry, 7, 359–365. doi:10.2174/187152007780618180.
Johnson, J. L., Beito, T. G., Krco, C. J., & Toft, D. O. (1994). Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Molecular and Cellular Biology, 14, 1956–1963.
Johnson, J. L., & Toft, D. O. (1994). A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. Journal of Biological Chemistry, 269, 24989–24993.
Kaur, D., Peng, J., Chinta, S. J., Rajagopalan, S., Di Monte, D. A., Cherny, R. A., et al. (2007). Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiology of Aging, 28, 907–913. doi:10.1016/j.neurobiolaging.2006.04.003.
Kitamura, Y., Inden, M., Miyamura, A., Kakimura, J., Taniguchi, T., & Shimohama, S. (2002). Possible involvement of both mitochondria- and endoplasmic reticulum-dependent caspase pathways in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neuroscience Letters, 333, 25–28. doi:10.1016/S0304-3940(02)00964-3.
Kopito, R. R. (2000). Aggresomes, inclusion bodies and protein aggregation. Trends in Cell Biology, 10, 524–530. doi:10.1016/S0962-8924(00)01852-3.
Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., et al. (1998). Ala30Pro mutation in the gene encoding a-synuclein in Parkinson’s disease. Nature Genetics, 18, 106–108. doi:10.1038/ng0298-106.
Lam, Y. A., Lawson, T. G., Velayutham, M., Zweier, J. L., & Pickart, C. M. (2002). A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature, 416, 763–767. doi:10.1038/416763a.
Lazebnik, Y. A., Kaufmann, S. H., Desnoyers, S., Poirier, G. G., & Earnshaw, W. C. (1994). Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature, 371, 346–347. doi:10.1038/371346a0.
Li, H., Zhu, H., Xu, C. J., & Yuan, J. (1998). Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell, 94, 491–501. doi:10.1016/S0092-8674(00)81590-1.
Li, L. J., Li, X., Ferrario, A., Rucker, N., Liu, E. S., Wong, S., et al. (1992). Establishment of a Chinese hamster ovary cell line that expresses grp78 antisense transcripts and suppresses A23187 induction of both GRP78 and GRP94. Journal of Cellular Physiology, 153, 575–582. doi:10.1002/jcp.1041530319.
Lindholm, D., Wootz, H., & Korhonen, L. (2006). ER stress and neurodegenerative diseases. Cell Death and Differentiation, 13, 385–392. doi:10.1038/sj.cdd.4401778.
Liu, H., Bowes, R. C., van de Water, B., 3rd, Sillence, C., Nagelkerke, J. F., & Stevens, J. L. (1997). Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. Journal of Biological Chemistry, 272, 21751–21759. doi:10.1074/jbc.272.35.21751.
Liu, H., Miller, E., van de Water, B., & Stevens, J. L. (1998). Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death. Journal of Biological Chemistry, 273, 12858–12862. doi:10.1074/jbc.273.21.12858.
Mattson, M. P. (2006). Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxidants Redox Signaling, 8, 1997–2006. doi:10.1089/ars.2006.8.1997.
McCormack, A. L., Thiruchelvam, M., Manning-Bog, A. B., Thiffault, C., Langston, J. W., Cory-Slechta, D. A., et al. (2002). Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiology of Disease, 10, 119–127. doi:10.1006/nbdi.2002.0507.
McCormack, A. L., & Di Monte, D. A. (2003). Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. Journal of Neurochemistry, 85, 82–86.
McCormack, A. L., Atienza, J. G., Johnston, L. C., Andersen, J. K., Vu, S., & Di Monte, D. A. (2005). Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. Journal of Neurochemistry, 93, 1030–1037. doi:10.1111/j.1471-4159.2005.03088.x.
Nakamura, T., & Lipton, S. A. (2008). Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxidants Redox Signaling, 10, 87–101. doi:10.1089/ars.2007.1858.
Paschen, W., & Frandsen, A. (2001). Endoplasmic reticulum dysfunction—a common denominator for cell injury in acute and degenerative diseases of the brain? Journal of Neurochemistry, 79, 719–725. doi:10.1046/j.1471-4159.2001.00623.x.
Peng, J., Mao, X. O., Stevenson, F. F., Hsu, M., & Andersen, J. K. (2004). The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. Journal of Biological Chemistry, 279, 32626–32632. doi:10.1074/jbc.M404596200.
Peng, J., Stevenson, F. F., Doctrow, S. R., & Andersen, J. K. (2005). Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: Implications for Parkinson disease. Journal of Biological Chemistry, 280, 29194–29198. doi:10.1074/jbc.M500984200.
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., et al. (1997). Mutation in the a-synuclein gene identified in families with Parkinson’s disease. Science, 276, 2045–2047. doi:10.1126/science.276.5321.2045.
Rajagopalan, S., & Andersen, J. K. (2001). Alpha synuclein aggregation: is it the toxic gain of function responsible for neurodegeneration in Parkinson’s disease? Mechanisms of Ageing and Development, 122, 1499–1510. doi:10.1016/S0047-6374(01)00283-4.
Rao, R. V., Hermel, E., Castro-Obregon, S., del Rio, G., Ellerby, L. M., Ellerby, H. M., et al. (2001). Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. Journal of Biological Chemistry, 276, 33869–33874. doi:10.1074/jbc.M102225200.
Rao, R. V., Castro-Obregon, S., Frankowski, H., Schuler, M., Stoka, V., Del Rio, G., et al. (2002a). Coupling endoplasmic reticulum stress to the cell death program. An APAF-1-independent intrinsic pathway. Journal of Biological Chemistry, 277, 21836–21842. doi:10.1074/jbc.M202726200.
Rao, R. V., Peel, A., Logvinova, A., del Rio, G., Hermel, E., Yokota, T., et al. (2002b). Coupling endoplasmic reticulum stress to the cell death program: Role of the ER chaperone GRP78. FEBS Letters, 514, 122–128.
Rao, R. V., & Bredesen, D. E. (2004). Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Current Opinion in Cell Biology, 16, 653–662. doi:10.1016/j.ceb.2004.09.012.
Rao, R. V., Ellerby, H. M., & Bredesen, D. E. (2004a). Coupling endoplasmic reticulum stress to the cell death program. Cell Death and Differentiation, 11, 372–380. doi:10.1038/sj.cdd.4401378.
Rao, R. V., Poksay, K. S., Castro-Obregon, S., Schilling, B., Row, R. H., Del Rio, G., et al. (2004b). Molecular components of a cell death pathway activated by endoplasmic reticulum stress. Journal of Biological Chemistry, 279, 177–187. doi:10.1074/jbc.M304490200.
Rao, R. V., Niazi, K., Mollahan, P., Mao, X., Crippen, D., Poksay, K. S., et al. (2006). Coupling endoplasmic reticulum stress to the cell-death program: A novel HSP90-independent role for the small chaperone protein p23. Cell Death and Differentiation, 13, 415–425. doi:10.1038/sj.cdd.4401761.
Reijonen, S., Putkonen, N., Norremolle, A., Lindholm, D., & Korhonen, L. (2008). Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Experimental Cell Research, 314, 950–960. doi:10.1016/j.yexcr.2007.12.025.
Riedl, S. J., & Salvesen, G. S. (2007). The apoptosome: Signalling platform of cell death. Nature Reviews. Molecular Cell Biology, 8, 405–413. doi:10.1038/nrm2153.
Rutkowski, D. T., & Kaufman, R. J. (2004). A trip to the ER: Coping with stress. Trends in Cell Biology, 14, 20–28. doi:10.1016/j.tcb.2003.11.001.
Ryu, E. J., Harding, H. P., Angelastro, J. M., Vitolo, O. V., Ron, D., & Greene, L. A. (2002). Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. Journal of Neuroscience, 22, 10690–10698.
Sitia, R., & Braakman, I. (2003). Quality control in the endoplasmic reticulum protein factory. Nature, 426, 891–894. doi:10.1038/nature02262.
Smith, W. W., Jiang, H., Pei, Z., Tanaka, Y., Morita, H., Sawa, A., et al. (2005). Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Human Molecular Genetics, 14, 3801–3811. doi:10.1093/hmg/ddi396.
Sokka, A. L., Putkonen, N., Mudo, G., Pryazhnikov, E., Reijonen, S., Khiroug, L., et al. (2007). Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. Journal of Neuroscience, 27, 901–908. doi:10.1523/JNEUROSCI.4289-06.2007.
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., & Goedert, M. (1997). Alpha-synuclein in Lewy bodies [letter]. Nature, 388, 839–840. doi:10.1038/42166.
Takahashi, R., Imai, Y., Hattori, N., & Mizuno, Y. (2003). Parkin and endoplasmic reticulum stress. Annals of the New York Academy of Sciences, 991, 101–106.
Uehara, T., Nakamura, T., Yao, D., Shi, Z. Q., Gu, Z., Ma, Y., et al. (2006). S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature, 441, 513–517. doi:10.1038/nature04782.
Wang, H. Q., & Takahashi, R. (2007). Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxidants Redox Signaling, 9, 553–561. doi:10.1089/ars.2006.1524.
Yamaguchi, H., & Wang, H. G. (2004). CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. Journal of Biological Chemistry, 279, 45495–45502. doi:10.1074/jbc.M406933200.
Yang, L., Sugama, S., Mischak, R. P., Kiaei, M., Bizat, N., Brouillet, E., et al. (2004). A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiology of Disease, 17, 250–259. doi:10.1016/j.nbd.2004.07.021.
Yang, W., & Tiffany-Castiglioni, E. (2005). The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: Relevance to the dopaminergic pathogenesis. Journal of Toxicology and Environmental Health. Part A, 68, 1939–1961. doi:10.1080/15287390500226987.
Yang, W., & Tiffany-Castiglioni, E. (2007). The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH-SY5Y cells. Journal of Toxicology and Environmental Health. Part A, 70, 1849–1857. doi:10.1080/15287390701459262.
Yang, W., & Tiffany-Castiglioni, E. (2008). Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: Involvement of p53 and mitochondria. Journal of Toxicology and Environmental Health. Part A, 71, 289–299. doi:10.1080/15287390701738467.
Yu, Z., Luo, H., Fu, W., & Mattson, M. P. (1999). The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: Suppression of oxidative stress and stabilization of calcium homeostasis. Experimental Neurology, 155, 302–314. doi:10.1006/exnr.1998.7002.
Zhou, W., Hurlbert, M. S., Schaack, J., Prasad, K. N., & Freed, C. R. (2000). Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Research, 866, 33–43. doi:10.1016/S0006-8993(00)02215-0.
Zhu, S., & Tytgat, J. (2004). Evolutionary epitopes of Hsp90 and p23: Implications for their interaction. FASEB Journal, 18, 940–947. doi:10.1096/fj.04-1570hyp.
Acknowledgments
We thank the members of the Bredesen laboratory and Andersen laboratory for helpful comments and discussions, and Molly Susag for administrative assistance. This work was supported by grants from the National Institutes of Health NS33376 to D.E.B. & R.V.R, and AG12282 to D.E.B.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chinta, S.J., Rane, A., Poksay, K.S. et al. Coupling Endoplasmic Reticulum Stress to the Cell Death Program in Dopaminergic Cells: Effect of Paraquat. Neuromol Med 10, 333–342 (2008). https://doi.org/10.1007/s12017-008-8047-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-008-8047-9