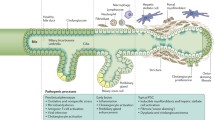
Abstract
A role of genetics in primary sclerosing cholangitis (PSC) development is now firmly established. A total of 16 risk genes have been reported at highly robust (“genome-wide”) significance levels, and ongoing efforts suggest that the list will ultimately be considerably longer. Importantly, this genetic risk pool so far accounts for less than 10 % of an estimated overall PSC susceptibility. The relative importance of genetic versus environmental factors (including gene-gene and gene-environment interactions) in remaining aspects of PSC pathogenesis is unknown, and other study designs than genome-wide association studies are needed to explore these aspects. For some of the loci, e.g. HLA and FUT2, distinct interacting environmental factors may exist, and working from the genetic associations may prove one valid path for determining the specific nature of environmental triggers. So far the biological implications for PSC risk genes are typically merely hypothesized based on previously published literature, and there is therefore a strong need for dedicated translational studies to determine their roles within the specific disease context of PSC. Apparently, most risk loci seem to involve in a subset of biological pathways for which genetic associations exist in a multitude of immune-mediated diseases, accounting for both inflammatory bowel disease as well as prototypical autoimmunity. In the present article, we will survey the current knowledge on PSC genetics with a particular emphasis on the pathophysiological insight potentially gained from genetic risk loci involving in this profound immunogenetic pleiotropy.


Similar content being viewed by others
References
Hirschfield GM, Karlsen TH, Lindor KD, Adams DH (2013) Primary sclerosing cholangitis. Lancet 382:1587–1599
Abdalian R, Heathcote EJ (2006) Sclerosing cholangitis: a focus on secondary causes. Hepatology 44:1063–1074
Karlsen TH, Boberg KM (2013) Update on primary sclerosing cholangitis. J Hepatol 59:571–582
Karlsen TH, Schrumpf E, Boberg KM (2010) Update on primary sclerosing cholangitis. Dig Liver Dis 42:390–400
Gotthardt D, Runz H, Keitel V et al (2008) A mutation in the canalicular phospholipid transporter gene, ABCB4, is associated with cholestasis, ductopenia, and cirrhosis in adults. Hepatology 48:1157–1166
Mendes FD, Jorgensen R, Keach J et al (2006) Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol 101:2070–2075
Bergquist A, Montgomery SM, Bahmanyar S et al (2008) Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 6:939–943
Bergquist A, Lindberg G, Saarinen S, Broome U (2005) Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol 42:252–256
Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H (1998) Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol 33:99–103
Ellinghaus D, Folseraas T, Holm K et al (2013) Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology 58:1074–1083
Folseraas T, Melum E, Rausch P et al (2012) Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol 57:366–375
Srivastava B, Mells GF, Cordell HJ et al (2012) Fine mapping and replication of genetic risk loci in primary sclerosing cholangitis. Scand J Gastroenterol 47:820–826
Liu JZ, Hov JR, Folseraas T et al (2013) Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet 45:670–675
Melum E, Franke A, Schramm C et al (2011) Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet 43:17–19
Karlsen TH, Franke A, Melum E et al (2010) Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 138:1102–1111
Karlsen TH, Melum E, Franke A (2010) The utility of genome-wide association studies in hepatology. Hepatology 51:1833–1842
Jostins L, Ripke S, Weersma RK et al (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124
Fausa O, Schrumpf E, Elgjo K (1991) Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis 11:31–39
Loftus EV Jr, Harewood GC, Loftus CG et al (2005) PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54:91–96
Jorgensen KK, Grzyb K, Lundin KE et al (2012) Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis 18:536–545
Saarinen S, Olerup O, Broome U (2000) Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol 95:3195–3199
Yoneno K, Hisamatsu T, Shimamura K et al (2013) TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 139:19–29
McMahan RH, Wang XX, Cheng LL et al (2013) Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem 288:11761–11770
Mells GF, Kaser A, Karlsen TH (2013) Novel insights into autoimmune liver diseases provided by genome-wide association studies. J Autoimmun 46:41–54
Cotsapas C, Voight BF, Rossin E et al (2011) Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet 7:e1002254
Horton R, Wilming L, Rand V et al (2004) Gene map of the extended human MHC. Nat Rev Genet 5:889–899
Ahmad T, Neville M, Marshall SE et al (2003) Haplotype-specific linkage disequilibrium patterns define the genetic topography of the human MHC. Hum Mol Genet 12:647–656
Degli-Esposti MA, Leaver AL, Christiansen FT, Witt CS, Abraham LJ, Dawkins RL (1992) Ancestral haplotypes: conserved population MHC haplotypes. Hum Immunol 34:242–252
Aly TA, Eller E, Ide A et al (2006) Multi-SNP analysis of MHC region: remarkable conservation of HLA-A1-B8-DR3 haplotype. Diabetes 55:1265–1269
Donaldson PT, Norris S (2001) Immunogenetics in PSC. Best Pract Res Clin Gastroenterol 15:611–627
Traherne JA (2008) Human MHC architecture and evolution: implications for disease association studies. Int J Immunogenet 35:179–192
Hov JR, Kosmoliaptsis V, Traherne JA et al (2011) Electrostatic modifications of the human leukocyte antigen-DR P9 peptide-binding pocket and susceptibility to primary sclerosing cholangitis. Hepatology 53:1967–1976
Hov JR, Lleo A, Selmi C et al (2010) Genetic associations in Italian primary sclerosing cholangitis: heterogeneity across Europe defines a critical role for HLA-C. J Hepatol 52:712–717
Karlsen TH, Boberg KM, Olsson M et al (2007) Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol 46:899–906
Rioux JD, Goyette P, Vyse TJ et al (2009) Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc Natl Acad Sci U S A 106:18680–18685
Parkes M, Cortes A, van Heel DA, Brown MA (2013) Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet 14:661–673
Karlsen TH (2012) A lecture on the genetics of primary sclerosing cholangitis. Dig Dis 30(Suppl 1):32–38
Karlsen TH, Kaser A (2011) Deciphering the genetic predisposition to primary sclerosing cholangitis. Semin Liver Dis 31:188–207
Henriksen, E.K.K., Melum, E., and Karlsen, T.H. (2014), Update on primary sclerosing cholangitis genetics. Curr Opin Gastroenterol In press
Hoyer KK, Dooms H, Barron L, Abbas AK (2008) Interleukin-2 in the development and control of inflammatory disease. Immunol Rev 226:19–28
Malek TR (2008) The biology of interleukin-2. Annu Rev Immunol 26:453–479
Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW (1995) Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3:521–530
Sebode M, Peiseler M, Franke B et al (2014) Reduced FOXP3 regulatory T cells in patients with primary sclerosing cholangitis are associated with IL2RA gene polymorphisms. J Hepatol 53(3):311–319
Navarro MN, Goebel J, Feijoo-Carnero C, Morrice N, Cantrell DA (2011) Phosphoproteomic analysis reveals an intrinsic pathway for the regulation of histone deacetylase 7 that controls the function of cytotoxic T lymphocytes. Nat Immunol 12:352–361
Dequiedt F, Kasler H, Fischle W et al (2003) HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity 18:687–698
Li B, Samanta A, Song X et al (2007) FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A 104:4571–4576
Hanna RN, Shaked I, Hubbeling HG et al (2012) NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res 110:416–427
Parra M, Kasler H, McKinsey TA, Olson EN, Verdin E (2005) Protein kinase D1 phosphorylates HDAC7 and induces its nuclear export after T-cell receptor activation. J Biol Chem 280:13762–13770
Matthews SA, Navarro MN, Sinclair LV, Emslie E, Feijoo-Carnero C, Cantrell DA (2010) Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem J 432:153–163
Navarro MN, Sinclair LV, Feijoo-Carnero C, Clarke R, Matthews SA, Cantrell DA (2012) Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells. Biochem J 442:649–659
Zhernakova A, van Diemen CC, Wijmenga C (2009) Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet 10:43–55
Wang J, Simonavicius N, Wu X et al (2006) Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 281:22021–22028
Oka S, Ota R, Shima M, Yamashita A, Sugiura T (2010) GPR35 is a novel lysophosphatidic acid receptor. Biochem Biophys Res Commun 395:232–237
Taniguchi Y, Tonai-Kachi H, Shinjo K (2006) Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett 580:5003–5008
Southern C, Cook JM, Neetoo-Isseljee Z et al (2013) Screening beta-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen 18:599–609
Fallarini S, Magliulo L, Paoletti T, de Lalla C, Lombardi G (2010) Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun 398:420–425
Imielinski M, Baldassano RN, Griffiths A et al (2009) Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet 41:1335–1340
Raelson JV, Little RD, Ruether A et al (2007) Genome-wide association study for Crohn's disease in the Quebec Founder Population identifies multiple validated disease loci. Proc Natl Acad Sci U S A 104:14747–14752
Goyette P, Lefebvre C, Ng A et al (2008) Gene-centric association mapping of chromosome 3p implicates MST1 in IBD pathogenesis. Mucosal Immunol 1:131–138
Yoshimura T, Yuhki N, Wang MH, Skeel A, Leonard EJ (1993) Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem 268:15461–15468
Liu QP, Fruit K, Ward J, Correll PH (1999) Negative regulation of macrophage activation in response to IFN-gamma and lipopolysaccharide by the STK/RON receptor tyrosine kinase. J Immunol 163:6606–6613
Cross-Disorder Group of the Psychiatric Genomics, C., Lee, S.H., Ripke, S. et al (2013) Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45:984–994
Zhuang Y, Cheng P, Weintraub H (1996) B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol Cell Biol 16:2898–2905
Bergqvist I, Eriksson M, Saarikettu J et al (2000) The basic helix-loop-helix transcription factor E2-2 is involved in T lymphocyte development. Eur J Immunol 30:2857–2863
Cisse B, Caton ML, Lehner M et al (2008) Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135:37–48
Wagner J, Catto-Smith AG, Cameron DJ, Kirkwood CD (2013) Pseudomonas infection in children with early-onset Crohn's disease: an association with a mutation close to PSMG1. Inflamm Bowel Dis 19:E58–59
Fasth AE, Cao D, van Vollenhoven R, Trollmo C, Malmstrom V (2004) CD28nullCD4+ T cells—characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand J Immunol 60:199–208
Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R (2001) CD4+CD28- costimulation-independent T cells in multiple sclerosis. J Clin Invest 108:1185–1194
Markle JG, Frank DN, Mortin-Toth S et al (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–1088
Spor A, Koren O, Ley R (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9:279–290
Yuan X, Waterworth D, Perry JR et al (2008) Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet 83:520–528
Chambers JC, Zhang W, Sehmi J et al (2011) Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet 43:1131–1138
Steinberg MW, Shui JW, Ware CF, Kronenberg M (2009) Regulating the mucosal immune system: the contrasting roles of LIGHT, HVEM, and their various partners. Semin Immunopathol 31:207–221
Sedy JR, Gavrieli M, Potter KG et al (2005) B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6:90–98
Smeets RL, Fleuren WW, He X et al (2012) Molecular pathway profiling of T lymphocyte signal transduction pathways; Th1 and Th2 genomic fingerprints are defined by TCR and CD28-mediated signaling. BMC Immunol 13:12
Hauser F, Deyle C, Berard D et al (2012) Macrophage-stimulating protein polymorphism rs3197999 is associated with a gain of function: implications for inflammatory bowel disease. Genes Immun 13:321–327
Bo X, Broome U, Remberger M, Sumitran-Holgersson S (2001) Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut 49:131–141
Muto A, Ochiai K, Kimura Y et al (2010) Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J 29:4048–4061
Hong SW, Kim S, Lee DK (2008) The role of Bach2 in nucleic acid-triggered antiviral innate immune responses. Biochem Biophys Res Commun 365:426–432
Dendrou CA, Plagnol V, Fung E et al (2009) Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat Genet 41:1011–1015
Hsu W, Zhang W, Tsuneyama K et al (2009) Differential mechanisms in the pathogenesis of autoimmune cholangitis versus inflammatory bowel disease in interleukin-2Ralpha(-/-) mice. Hepatology 49:133–140
Hanna RN, Carlin LM, Hubbeling HG et al (2011) The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 12:778–785
Kasler HG, Young BD, Mottet D et al (2011) Histone deacetylase 7 regulates cell survival and TCR signaling in CD4/CD8 double-positive thymocytes. J Immunol 186:4782–4793
Zhernakova A, Elbers CC, Ferwerda B et al (2010) Evolutionary and functional analysis of celiac risk loci reveals SH2B3 as a protective factor against bacterial infection. Am J Hum Genet 86:970–977
Maier LM, Hafler DA (2009) Autoimmunity risk alleles in costimulation pathways. Immunol Rev 229:322–336
Dequiedt F, Van Lint J, Lecomte E et al (2005) Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J Exp Med 201:793–804
Clark K, MacKenzie KF, Petkevicius K et al (2012) Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc Natl Acad Sci U S A 109:16986–16991
Wagner J, Sim WH, Ellis JA et al (2010) Interaction of Crohn's disease susceptibility genes in an Australian paediatric cohort. PLoS One 5:e15376
Civelek M, Lusis AJ (2014) Systems genetics approaches to understand complex traits. Nat Rev Genet 15:34–48
Acknowledgements
We thank Tor Halland (TorDesign) and Øystein Horgmo (University of Oslo) for assistance in preparing Fig. 1 and Fig. 2, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Folseraas, T., Liaskou, E., Anderson, C.A. et al. Genetics in PSC: What Do the “Risk Genes” Teach Us?. Clinic Rev Allerg Immunol 48, 154–164 (2015). https://doi.org/10.1007/s12016-014-8417-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-014-8417-z