Abstract
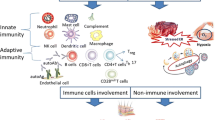
Skeletal muscle is the target tissue of immunoflogistic processes in patients affected with idiopathic inflammatory myopathies (IIM). IIM are classified into three major forms: polymyositis (PM), dermatomyositis (DM), and inclusion body myositis. Recent data suggest that, in the major subsets of myositis, antigens in muscles drive a B-cell antigen-specific immune response. Moreover, some non-immunological mechanisms have been advocated. In this regard, an increased expression of Jo-1 and Mi-2 in muscle biopsies from PM and DM patients compared to normal muscle has been demonstrated; these candidate autoantigens in myositis are expressed at high levels in regenerating muscle cells rather than in mature myotubes. Myositis autoantigen upregulation has also been observed in neoplastic tissues, thus representing a potential link between cancer and autoimmunity in myositis. Myositis-specific autoantibodies (MSA) are disease markers and target intracellular proteins involved in key processes such as translocation and nuclear transcription. Myositis target antigens encompass aminoacyl-tRNA synthetases, the Mi-2 helicase/histone deacetylase protein complex, the signal recognition particle ribonucleoprotein, together with novel target antigens including p155/140, CADM-140, and SAE. Despite their high specificity for autoimmune myositis, MSA target non-muscle restricted proteins ubiquitary to all cell types, making the specific muscle involvement difficult to explain. Non-immunological mechanisms also seem to contribute to the pathogenesis of IIM; activation of endoplasmic reticulum stress response due to muscle regeneration and inflammation but independent to MHC-1 up-regulation has been recently reported in patients with myositis.


Similar content being viewed by others
References
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first part). N Engl J Med 292:344–347
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second part). N Engl J Med 292:403–407
Dalakas MC, Hohlfeld R (2003) Polymyositis and dermatomyositis. Lancet 362:971–982
Dalakas MC (1992) Clinical, immunopathologic and therapeutic considerations of inflammatory myopathies. Clin Neuropharmacol 15:327–351
Prieto S, Grau JM (2010) The geoepidemiology of autoimmune muscle disease. Autoimmun Rev 9:A330–A334
Mathiesen PR, Zak M, Heilin T, Nielsen SM (2010) Clinical features and outcome in a Danish cohort of juvenile dermatomyositis patients. Clin Exp Rheumatol 28:782–789
Dalakas MC (1998) Molecular immunology and genetics of inflammatory muscle diseases. Arch Neurol 55:1509–1512
Grundtman C, Lundberg IE (2009) Vascular involvement in the pathogenesis of idiopathic inflammatory myopathies. Autoimmunity 42:615–626
Greenberg SA (2007) A gene expression approach to study perturbed pathways in myositis. Curr Opin Rheumatol 19:536–541
Lundberg IE, Helmers SB (2010) The type I interferon system in idiopathic inflammatory myopathies. Autoimmunity 43:239–243
Chevrel G, Page G, Miossec P (2006) Novel aspects on the contribution of T cells and dendritic cells in the pathogenesis of myositis. Autoimmunity 39:171–176
Salomonsson S, Lundberg IE (2006) Cytokines in idiopathic inflammatory myopathies. Autoimmunity 39:177–190
Casciola-Rosen L, Nagaraij K, Plots P, Wang K, Levine S, Gabrielson E et al (2005) Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med 201:591–601
Carraro U, Rossini K, Mayr W, Kern H (2005) Muscle fiber regeneration in human permanent lower motoneuron denervation: relevance to safety and effectiveness of FES-training, which induces muscle recovery in SCI subjects. Artif Organs 29:187–191
Wagers AJ, Concoy IM (2005) Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122:659–667
Cantini M, Carraro U (1996) Control of cell proliferation by macrophage-myoblast interactions. Basic Appl Myol 6:485–490
Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Planquet A et al (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. JEM 204:1057–1069
Dhawan J, Rando TA (2005) Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15:666–673
Zampieri S, Biral D, Adami N, Ghirardello A, Rampudda M, Tonello M et al (2008) Expression of myositis specific autoantigens during postnatal myogenesis. Neurol Res 30:145–148
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288:R345–R353
Hill CL, Zhang Y, Sigurgeirsson B et al (2001) Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357:96–100
Sigurgeirsson B, Lindelöf B, Edhag O, Allander E (1992) Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med 326:363–367
Buchbinder R, Forbes A, Hall S, Dennett X, Giles G (2001) Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med 134:1087–1095
Wakata N, Kurihara T, Saito E, Kinoshita M (2002) Polymyositis and dermatomyositis associated with malignancy: a 30-year retrospective study. Int J Dermatol 41:729–734
Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y (2001) Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis 60:433–440
Chakravarty E, Genovese MC (2003) Rheumatic syndromes associated with malignancy. Curr Opin Rheumatol 15:35–43
Raccanelli V, Prete M, Minoia C, Favoino E, Perosa F (2008) Rheumatic disorders as paraneoplastic syndromes. Autoimmun Rev 7:352–358
András C, Csiki Z, Ponyi A, Illés A, Dankó K (2006) Paraneoplastic rheumatic syndromes. Rheumatol Int 26:376–382
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Eisenlohr LC, Rothstein JL (2006) Oncogenic inflammation and autoimmune disease. Autoimmun Rev 6:107–114
Levine SM (2006) Cancer and myositis: new insights into an old association. Curr Opin Rheumatol 18:620–624
Russel JP, Engiles JB, Rothstein JL (2004) Proinflammatory mediators and genetic background in oncogene mediated tumor progression. J Immunol 172:4059–4067
Zampieri S, Doria A, Adami N, Biral N, Vecchiato M, Savastano S et al (2010) Subclinical myopathy in patients affected with early stage colorectal cancer at disease onset: evidence from skeletal muscle biopsies. Neurol Res 32:20–25
Zampieri S, Valente M, Adami N, Corbianco S, Doria A, Biral D et al (2009) Subclinical myopathy in patients affected with early stage colorectal cancer at disease onset: no evidence of inflammatory cells infiltration in the skeletal muscle biopsies harvested during diagnostic laparoscopy. Basic Appl Myol 19:247–252
Zampieri S, Valente M, Adami N, Biral N, Ghirardello A, Rampudda M et al (2010) Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev 9:449–453
Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH et al (1991) A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine 70:360–374
Brouwer R, Hengstmam GJ, Vree Egberts W, Ehrfeld H, Bozic B, Ghirardello A et al (2001) Autoantibodies profiles in the sera of European patients with myositis. Ann Rheum Dis 60:116–123
Ghirardello A, Zampieri S, Tarricone E, Iaccarino L, Bendo R, Briani C et al (2006) Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 39:217–221
Chinoy H, Fertig N, Oddis CV, Ollier WER, Cooper RG (2007) The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis 66:1345–1349
Troyanov Y, Targoff IN, Tremblay JL, Gauler JR, Raymand Y, Senecal JL (2005) Novel classification of idiopathic inflammatory myopathies based on overlap features and autoantibodies. Medicine 84:231–249
Sordet C, Goetz J, Sibilia J (2006) Contribution of autoantibodies to the diagnosis and nosology of inflammatory muscle disease. J Bone Spine 73:646–654
Koenig M, Fritzler MJ, Targoff IN, Troyanov Y, Senecal JL (2007) Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes. Arthritis Res Ther 9:R78
Franceschini F, Cavazzana I, Generali D, Quinzanini M, Ghirardello A, Doria A et al (2002) Anti-Ku Antibodies in connective tissue diseases: clinical and serological evaluation of 14 patients. J Rheumatol 29:1393–1397
Rutjes SA, Vree Egberts WTM, Jongen P, van den Hoogen F, Prujin GJM, van Venrooij WJ (1997) Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 109:32–40
Defendenti C, Atzeni F, Spina MF, Grosso S, Cereda A, Guercilena G et al (2010) Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun Rev, Sep 18 (Epub ahead of print) PMID: 20854935
Váncsa A, Gergely L, Ponyi A, Lakos G, Nemeth J, Szodoray P et al (2010) Myositis-specific and myositis-associated antibodies in overlap myositis in comparison to primary dermatopolymyositis. Relevance for clinical classification: retrospective study of 169 patients. Joint Bone Spine 77:125–130
Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A (2005) Anti-Jo-1 antibodies. Autoimmunity 38:73–78
Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Bendo R, Gambari PF et al (2005) Anti-Mi-2 autoantibodies. Autoimmunity 38:79–83
Hengstman GJD, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J et al (2006) Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2β antigen. Ann Rheum Dis 65:242–245
Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J et al (2006) Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis 65:1635–1638
Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T et al (2005) Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52:1571–1576
Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura T et al (2007) Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology 46:25–28
Betteridge Z, Gunawardena H, North J, Slinn J, McHugh N (2007) Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum 56:3132–3137
Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL (2010) A novel autoantibody recognizing 200-kd and 100-kd proteins is Associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 62:2757–2766
Betteridge ZE, Gunawardena H, McHugh NJ (2009) Pathogenic mechanisms of disease in myositis: autoantigens as clues. Curr Opin Rheumatol 21:604–609
Gunawardena H, Betteridge ZE, McHugh NJ (2008) Newly identified autoantibodies: relationship to idiopathic inflammatory myopathy subsets and pathogenesis. Curr Opin Rheumatol 20:675–680
Hashish L, Trieu EP, Sadanandan P, Targoff IN (2005) Identification of autoantibodies to tyrosyl-tRNA synthetase in dermatomyositis with features consistent with antisynthetase syndrome (abstract). Arthritis Rheum 52:S312
Betteridge Z, Gunawardena H, North J, Slinn J, McHugh N (2007) Antisynthetase syndrome: a new autoantibody to phenylalanyl transfer RNA synthetase (anti-Zo) associated with polymyositis and interstitial pneumonia. Rheumatology 46:1005–1008
Zheng WJ, Wei W, Tang FL (2003) Clinical features and misdiagnosis of anti-Jo-1 syndrome: analysis of 33 cases. Zhongua Yi Xue Zhi 83:1565–1568
Yamasaki Y, Yamada H, Nozaki T, Akaogi J, Nichols C, Lyons R et al (2006) Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 54:2004–2009
Targoff IN, Reichlin M (1985) The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum 28:796–803
Nilasena DS, Trieu EP, Targoff IN (1995) Analysis of the Mi-2 autoantigen of dermatomyositis. Arthritis Rheum 38:123–128
Wang HB, Zhang Y (2001) Mi2, an auto-antigen for dermatomyositis, is an ATP-dependent nucleosome remodeling factor. Nucleic Acid Res 15:2517–2521
Mierau R, Dick T, Bartz-Bazzanella P, Keller E, Albert ED, Genth E (1996) Strong association of dermatomyositis specific Mi-2 autoantibodies with a tryptophan at position 9 of the HLA-DRβ chain. Arthritis Rheum 39:868–7636
Walter P, Blobel G (1982) Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 299:691–698
Romisch K, Miller FW, Dobberstein B, High S (2006) Human autoantibodies against the 54 kDa protein of the signal recognition particle block function at multiple stages. Arthritis Res Ther 8:R39
Reeves WH, Ngam SK, Blobel G (1986) Humna autoantibodies reactive with the signal recognition particle. Proc Natl Acad Sci USA 83:9507–9511
Whelan BR, Isenberg DA (2009) Poor response of anti-SRP-positive idiopathic immune myositis to B-cell depletion. Rheumatology 48:594–595
Miller T, Al-Lozi MT, Pestronk A (2002) Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry 73:420–428
Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV (2004) Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum 50:209–215
Suzuki S, Satoh T, Sato S, Otomo M, Hirayama Y, Sato H et al (2008) Clinical utility od anti-signal recognition particle antibody in the differential diagnosis of myopathies. Rheumatology 47:1539–1542
Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O’Hanlon TP et al (2006) A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum 54:3682–3689
Targoff IN, Trieu EP, Levy-Neto M, Prasertsuntarsai T (2006) Autoantibodies to transcriptional intermediary factor 1-gamma (TIF1-g) in dermatomyositis (abstract). Arthritis Rheum 54:S518
Trallero-Araguas E, Labrador-Horrillo M, Selva-O’Callaghan A, Martinez MA, Martinez-Gomez X, Palou E et al (2010) Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine 89:47–52
Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T (2010) Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology 49:3–1726
Sato S, Hoshino K, Satoh T, Suwa A, Hirakata M (2008) MDA5 (melanoma differentiation-associated gen 5) as an autoantigen recognized by anti-CADM-140 antibody in patients with clinically amyopathic dermatomyositis (abstract). Arthritis Rheum 58:S923
Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WE, Cooper RG et al (2009) Clinical and HLA-class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK adult-onset Caucasian myositis. Ann Rheum Dis 68:1621–1625
Ghirardello A, Bendo R, Rampudda ME, Bassi N, Zampieri S, Doria A (2009) Commercial blot assays in the diagnosis of systemic rheumatic diseases. Autoimmun Rev 8:645–649
Ghirardello A, Rampudda M, Ekholm L, Bassi N, Tarricone E, Zampieri S et al (2010) Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology 49(12):2370–2374
Ronnelid J, Barbasso Helmers S, Storfors H, Grip K, Ronnblom L, Frank-Larsson K et al (2009) Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun Rev 9:58–61
Trieu EP, Gross JK, Targoff IN (2009) Immunoprecipitation-Western blot for proteins of low abundance, Chapter 28. In: Kurien BT, Scofield RH (eds) Methods in molecular biology, protein blotting and detection, vol 536. Humana, New York
Henriques-Pons A, Nagaraju K (2009) Non-immune mechanisms of muscle damage in myositis: role of the reticulum stress response and autophagy in the disease pathogenesis. Curr Opin Rheumatol 21:581–587
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1383–1388
Vattemi G, King Engel W, McFerrin J, Askanas V (2004) Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol 164:1–7
Vattemi G, Nogalska A, King Engel W, D’Agostino C, Checler F, Askanas V (2009) Amyloid-b42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis. Acta Neuropathol 117:569–574
Fratta P, Engel WK, Van Leeuwen FV, Hol EM, Vattemi G, Askanas V (2004) Mutant ubiquitin UBB+1 is accumulated in sporadic inclusion-body myositis muscle fibers. Neurology 63:1114–1117
Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R et al (2005) Activation of the endoplasmic reticulum response in autoimmune myositis. Arthritis Rheum 52:824–835
Nagaraju K (2005) Role of major histocompatibility complex class I molecules in autoimmune myositis. Curr Opin Rheumatol 17:725–730
Sallum AM, Kiss MH, Silva CA, Wakamatsu A, Sachetti S, Lotufo S et al (2009) MHC class I and II expression in juvenile dermatomyositis skeletal muscle. Clin Exp Rheumatol 27:519–526
Creus KK, De Poepe B, De Bleecker JL (2009) Idiopathic inflammatory myopathies and the classical NK-kappaB complex: current insights and implications for therapy. Autoimmun Rev 8:627–631
Vitadello M, Doria A, Tarricone E, Ghirardello A, Gorza L (2010) Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and sistemic inflammation. Arthritis Res Ther 12:R52
Nakanishi K, Sudo T, Morishima N (2005) Endoplasmic reticulum stress signalling transmitted by ATF6 mediates apoptosis during muscle development. J Cell Biol 169:555–560
Tarricone E, Ghirardello A, Zampieri A, Rampudda ME, Doria A, Gorza L (2006) Cell stress-response in skeletal muscle myofibers. Ann NY Acad Sci 1069:472–476
Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z (2003) Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. PNAS 100:15824–15829
Gorza L, Vitadello M (2000) Reduced amount of the glucose-regulated protein GRP94 in skeletal myoblasts results in loss of fusion competence. FASEB J 14:461–475
Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Gorza L (2009) A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol 107:549–557
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghirardello, A., Zampieri, S., Tarricone, E. et al. Cutting Edge Issues in Polymyositis. Clinic Rev Allerg Immunol 41, 179–189 (2011). https://doi.org/10.1007/s12016-010-8238-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-010-8238-7