Abstract
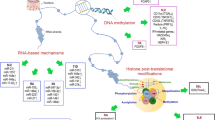
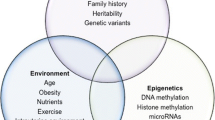
The decline in immunocompetence with age is accompanied by the increase in the incidence of autoimmune diseases. Aging of the immune system, or immunosenescence, is characterized by a decline of both T and B cell function, and paradoxically the presence of low-grade chronic inflammation. There is growing evidence that epigenetics, the study of inherited changes in gene expression that are not encoded by the DNA sequence itself, changes with aging. Interestingly, emerging evidence suggests a key role for epigenetics in human pathologies, including inflammatory and neoplastic disorders. Here, we will review the potential mechanisms that contribute to the increase in autoimmune responses in aging. In particular, we will discuss how epigenetic alterations, especially DNA methylation and histone acetylation, are accumulated during aging and how these events contribute to autoimmunity risk.
Similar content being viewed by others
References
Hasler P, Zouali M (2005) Immune receptor signaling, aging, and autoimmunity. Cell Immunol 233:102–108
Franceschi C, Bonafe M, Valensin S et al (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 908:244–254
Sansoni P, Vescovini R, Fagnoni F et al (2008) The immune system in extreme longevity. Exp Gerontol 43:61–65
Bandyopadhyay D, Medrano EE (2003) The emerging role of epigenetics in cellular and organismal aging. Exp Gerontol 38:1299–1307
Goronzy JJ, Weyand CM (2003) Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity—catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther 5:225–234
Liuzzo G, Biasucci LM, Trotta G et al (2007) Unusual CD4+ CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol 50:1450–1458
Weyand CM, Fulbright JW, Goronzy JJ (2003) Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol 38:833–841
Stacy S, Krolick KA, Infante AJ, Kraig E (2002) Immunological memory and late onset autoimmunity. Mech Ageing Dev 123:975–985
Johnson SA, Cambier JC (2004) Ageing, autoimmunity and arthritis: senescence of the B cell compartment—implications for humoral immunity. Arthritis Res Ther 6:131–139
Kline GH, Hayden TA, Klinman NR (1999) B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol 162:3342–3349
Johnson TE (2006) Recent results: biomarkers of aging. Exp Gerontol 41:1243–1246
Krabbe KS, Pedersen M, Bruunsgaard H (2004) Inflammatory mediators in the elderly. Exp Gerontol 39:687–699
Walston J, McBurnie MA, Newman A et al (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162:2333–2341
Maggio M, Guralnik JM, Longo DL, Ferrucci L (2006) Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol, A Biol Sci Med Sci 61:575–584
Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY (2007) Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev 21:3244–3257
Helenius M, Hanninen M, Lehtinen SK, Salminen A (1996) Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J 318:603–608
Kasapis C, Thompson PD (2005) The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 45:1563–1569
Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE (2003) The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc 51:1125–1130
Deon D, Ahmed S, Tai K et al (2001) Cross-talk between IL-1 and IL-6 signaling pathways in rheumatoid arthritis synovial fibroblasts. J Immunol 167:5395–5403
Esteller M (2006) The necessity of a human epigenome project. Carcinogenesis 27:1121–1125
Issa JP (2003) Age-related epigenetic changes and the immune system. Clin Immunol 109:103–108
Falls JG, Pulford DJ, Wylie AA, Jirtle RL (1999) Genomic imprinting: implications for human disease. Am J Pathol 154:635–647
Feinberg AP, Cui H, Ohlsson R (2002) DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol 12:389–398
Csankovszki G, Nagy A, Jaenisch R (2001) Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. J Cell Biol 153:773–784
Brockdorff N (2002) X-chromosome inactivation: closing in on proteins that bind Xist RNA. Trends Genet 18:352–358
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33:245–254
Fraga MF, Ballestar E, Paz MF et al (2005) Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 102:10604–10609
Mays-Hoopes L, Chao W, Butcher HC, Huang RC (1986) Decreased methylation of the major mouse long interspersed repeated DNA during aging and in myeloma cells. Dev Genet 7:65–73
Richardson B (2003) Impact of aging on DNA methylation. Ageing Res Rev 2:245–261
Wilson VL, Smith RA, Ma S, Cutler RG (1987) Genomic 5-methyldeoxycytidine decreases with age. J Biol Chem 262:9948–9951
Bollati V, Schwartz J, Wright R et al (2008) Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev 130:234–239
Casillas MA, Lopatina N, Andrews LG, Tollefsbol TO (2003) Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem 252:33
Lopatina N, Haskell JF, Andrews LG, Poole JC, Saldanha S, Tollefsbol T (2002) Differential maintenance and de novo methylating activity by three DNA methyltransferases in aging and immortalized fibroblasts. J Cell Biochem 84:324–334
Yung RL, Julius A (2008) Epigenetics, aging, and autoimmunity. Autoimmunity 41:329–335
Yoon YS, Choo JH, Yoo T, Kang K, Chung JH (2007) RhoB is epigenetically regulated in an age- and tissue-specific manner. Biochem Biophys Res Commun 362:164–169
Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH (2002) Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J Biol Chem 277:39195–39201
Kawakami K, Nakamura A, Ishigami A, Goto S, Takahashi R (2008) Age-related difference of site-specific histone modifications in rat liver. Biogerontology 10:415–421
Bennett-Baker PE, Wilkowski J, Burke DT (2003) Age-associated activation of epigenetically repressed genes in the mouse. Genetics 165:2055–2062
Fu VX, Dobosy JR, Desotelle JA et al (2008) Aging and cancer-related loss of insulin-like growth factor 2 imprinting in the mouse and human prostate. Cancer Res 68:6797–6802
Foley DL, Craig JM, Morley R et al (2009) Prospects for epigenetic epidemiology. Am J Epidemiol 169:389–400
Liu L, Wylie RC, Andrews LG, Tollefsbol TO (2003) Aging, cancer and nutrition: the DNA methylation connection. Mech Ageing Dev 124:989–998
Ross SA, Milner JA (2007) Epigenetic modulation and cancer: effect of metabolic syndrome? Am J Clin Nutr 86:s872–877
Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M (2005) Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4:119–125
Cooney CA, Dave AA, Wolff GL (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132:2393S–2400S
Wolff GL, Kodell RL, Moore SR, Cooney CA (1998) Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12:949–957
Cropley JE, Suter CM, Beckman KB, Martin DI (2006) Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci USA 103:17308–17312
Waterland RA, Lin JR, Smith CA, Jirtle RL (2006) Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet 15:705–716
Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC (2008) Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr 100:278–282
Hollingsworth JW, Maruoka S, Boon K et al (2008) In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest 118:3462–3469
Heijmans BT, Tobi EW, Stein AD et al (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 105:17046–17049
Feinberg AP, Ohlsson R, Henikoff S (2006) The epigenetic progenitor origin of human cancer. Nat Rev Genet 7:21–33
Robert MF, Morin S, Beaulieu N et al (2003) DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet 33:61–65
Laird PW, Jaenisch R (1994) DNA methylation and cancer. Hum Mol Genet 3:1487–1495
Lujambio A, Esteller M (2009) How epigenetics can explain human metastasis: a new role for microRNAs. Cell Cycle 8:377–382
Kato M, Slack FJ (2008) microRNAs: small molecules with big roles—C. elegans to human cancer. Biol Cell 100:71–81
Boehm M, Slack F (2005) A developmental timing microRNA and its target regulate life span in C. elegans. Science 310:1954–1957
Stanczyk J, Pedrioli DM, Brentano F et al (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58:1001–1009
Nakasa T, Miyaki S, Okubo A et al (2008) Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 58:1284–1292
Pallis M, Robins A, Powell R (1993) Quantitative analysis of lymphocyte CD11a using standardized flow cytometry. Scand J Immunol 38:559–564
Lu Q, Kaplan M, Ray D et al (2002) Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum 46:1282–1291
Richardson BC, Strahler JR, Pivirotto TS et al (1992) Phenotypic and functional similarities between 5-azacytidine-treated T cells and a T cell subset in patients with active systemic lupus erythematosus. Arthritis Rheum 35:647–662
Yung R, Powers D, Johnson K et al (1996) Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupus like disease in syngeneic mice. J Clin Invest 97:2866–2871
Kevil CG, Hicks MJ, He X et al (2004) Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am J Pathol 165:609–616
Zhang Z, Deng C, Lu Q, Richardson B (2002) Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech Ageing Dev 123:1257–1268
Bruniquel D, Schwartz RH (2003) Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol 4:235–240
Yano S, Ghosh P, Kusaba H, Buchholz M, Longo DL (2003) Effect of promoter methylation on the regulation of IFN-gamma gene during in vitro differentiation of human peripheral blood T cells into a Th2 population. J Immunol 171:2510–2516
Saccani S, Natoli G (2002) Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev 16:2219–2224
Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T (2008) Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev 7:83–105
Hodge DR, Cho E, Copeland TD et al (2007) IL-6 enhances the nuclear translocation of DNA cytosine-5-methyltransferase 1 (DNMT1) via phosphorylation of the nuclear localization sequence by the AKT kinase. Cancer Genomics Proteomics 4:387–398
Croonquist PA, Van Ness B (2005) The polycomb group protein enhancer of zeste homolog 2 (EZH2) is an oncogene that influences myeloma cell growth and the mutant ras phenotype. Oncogene 24:6269
Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC (2009) Stronger inflammatory/cytotoxic T cell response in women identified by microarray analysis. Genes Immun 10:509–516
Ray D, Wu A, Wilkinson JE et al (2006) Aging in heterozygous Dnmt1-deficient mice: effects on survival, the DNA methylation genes, and the development of amyloidosis. J Gerontol A Biol Sci Med Sci 61:115–124
Yung R, Ray D, Eisenbraun JK et al (2001) Unexpected effects of a heterozygous dnmt1 null mutation on age-dependent DNA hypomethylation and autoimmunity. J Gerontol, A Biol Sci Med Sci 56:B268–276
Li E, Bestor TH, Jaenisch R (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69:915–926
Takeda T, Hosokawa M, Takeshita S et al (1981) A new murine model of accelerated senescence. Mech Ageing Dev 17:183–194
Chang KT, Min KT (2002) Regulation of lifespan by histone deacetylase. Ageing Res Rev 1:313–326
Zhao Y, Sun H, Lu J et al (2005) Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol 208:697–705
Kypreou KP, Sourlingas TG, Sekeri-Pataryas KE (2004) Age-dependent response of lymphocytes in the induction of the linker histone variant, H1 degrees and histone H4 acetylation after treatment with the histone deacetylase inhibitor, trichostatin A. Exp Gerontol 39:469–479
Tsapali DS, Sekeri-Pataryas KE, Sourlingas TG (2000) Study of the H1 linker histone variant, H1o, during the in vitro aging of human diploid fibroblasts. Ann NY Acad Sci 908:336–340
Happel N, Doenecke D, Sekeri-Pataryas KE, Sourlingas TG (2008) H1 histone subtype constitution and phosphorylation state of the ageing cell system of human peripheral blood lymphocytes. Exp Gerontol 43:184–199
Wang L, Tao R, Hancock WW (2009) Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol 87:195–202
Lages CS, Suffia I, Velilla PA et al (2008) Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol 181:1835–1848
Sharma S, Dominguez AL, Lustgarten J (2006) High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol 177:8348–8355
Dejaco C, Duftner C, Schirmer M (2006) Are regulatory T-cells linked with aging? Exp Gerontol 41:339–345
Haigis MC, Guarente LP (2006) Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20:2913–2921
Mostoslavsky R, Chua KF, Lombard DB et al (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329
Michishita E, McCord RA, Berber E et al (2008) SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452:492–496
Kawahara TL, Michishita E, Adler AS et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136:62–74
Adler AS, Kawahara TL, Segal E, Chang HY (2008) Reversal of aging by NF kappa B blockade. Cell Cycle 7:556–559
Sequeira J, Boily G, Bazinet S et al (2008) sirt1-null mice develop an autoimmune-like condition. Exp Cell Res 314:3069–3074
Arnheim N, Calabrese P (2009) Understanding what determines the frequency and pattern of human germ line mutations. Nat Rev Genet 10:478–488
Barros SP, Offenbacher S (2009) Epigenetics: connecting environment and genotype to phenotype and disease. J Dent Res 88:400–408
Figueiredo LM, Cross GA, Janzen CJ (2009) Epigenetic regulation in African trypanosomes: a new kid on the block. Nat Rev Microbiol 7:504–513
Hewagama A, Richardson B (2009) The genetics and epigenetics of autoimmune diseases. J Autoimmun 33:3–11
Invernizzi P (2009) Future directions in genetic for autoimmune diseases. J Autoimmun 33:1–2
Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M (2009) Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun 33:12–16
Larizza D, Calcaterra V, Martinetti M (2009) Autoimmune stigmata in Turner syndrome: when lacks an X chromosome. J Autoimmun 33:25–30
Persani L, Rossetti R, Cacciatore C, Bonomi M (2009) Primary ovarian insufficiency: X chromosome defects and autoimmunity. J Autoimmun 33:35–41
Sawalha AH, Harley JB, Scofield RH (2009) Autoimmunity and Klinefelter's syndrome: when men have two X chromosomes. J Autoimmun 33:31–34
Wells AD (2009) New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol 182:7331–7341
Zernicka-Goetz M, Morris SA, Bruce AW (2009) Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet 10:467–477
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work is supported by the NIH (RO1AG020628; RO1AR42525; RY), the VA Ann Arbor Health System (VA Merit Review; RY), and by the NIH-NIA (AG024824, University of Michigan Claude Pepper Older Americans Independence Center; AG).
Rights and permissions
About this article
Cite this article
Grolleau-Julius, A., Ray, D. & Yung, R.L. The Role of Epigenetics in Aging and Autoimmunity. Clinic Rev Allerg Immunol 39, 42–50 (2010). https://doi.org/10.1007/s12016-009-8169-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-009-8169-3