Abstract
The first responsibility for protection against microbial infection rests on the normal function of the innate immune system. This system establishes an antimicrobial barrier, recognizes attempts to breach this barrier, and responds rapidly to danger, all based on an innate defense system. Here, we review this system as it applies to mammalian skin, highlighting how a physical, cellular, and chemical barrier is formed to resist infection. When challenged, the diverse cellular components of the skin recognize the nature of the challenge and respond with an appropriate antimicrobial program including the release of antimicrobial peptides and, when necessary, recruitment and coordination with adaptive immune responses. Recent insights into these processes have advanced the understanding of disease pathogenesis and provided new therapeutic options for a variety of skin diseases.
Similar content being viewed by others
Introduction
Current understanding of cutaneous immune defense mechanisms has developed into the recognition of two coordinated systems by which our bodies defend against infection and disease; innate and adaptive immunity. Innate immunity involves a collection of preexisting, nonspecific anatomical, secretory, and cellular mechanisms to combat infection. This is in contrast to specific or adaptive immunity that reacts following education by a specific immune stimulus (antigen). Although each system has distinct responsibilities, neither is effective alone and each is influenced by the other. This review will serve as an introduction to the innate system, a relatively recently recognized aspect of human immunity whose relevance to human disease is increasingly becoming appreciated.
Innate immunity is an evolutionarily ancient part of our defense system that is common among plants and animals, vertebrates and invertebrates. It provides the first line of defense against infection and typically acts as a broad-spectrum and rapid defense mechanism to clear pathogens or reduce their spread. It works with a limited repertoire of recognition molecules. Adaptive immunity, on the other hand, only exists in vertebrates. It is highly adaptive and provides specific recognition of foreign antigens, leading to immunological memory. Only if innate immunity is unable to control the pathogen will the adaptive immune system be fully activated. Because activation of the adaptive immune system can require several days, the innate immune system effectively buys the body time until the slower effector molecules of both the innate and the adaptive system appear to fight.
Historically, innate and adaptive immunity have been studied and considered as separate entities. Today, however, synergistic and interfacing parts of the two systems have been appreciated. There are now several mechanisms known by which the innate immune system instructs and directs adaptive immune responses [1]. Understanding this, and the distinct responsibilities of the innate immune system, is at the forefront of current basic research efforts in innate immunology.
Innate Immunity
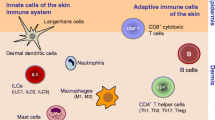
Being the first line of defense against pathogen attack, the innate immune system responds to a microbe in two different ways: (1) It recognizes invading pathogens and distinguishes pathogenic microorganisms from nonpathogens. (2) It starts a coordinated physiological response to the offenders producing substances that can destroy them directly. Innate immunity consists of three major sets of elements: the physical/anatomical barriers, the cellular components, and the chemical/secretory molecules (Fig. 1).
The innate immune response in skin as the first line of defense against pathogen attacks consists of three major sets of elements: the physical/anatomical barriers, the cellular components, and the chemical/secretory molecules. In response to a pathogen, innate immunity (1) releases antimicrobial agents, (2) induces inflammatory mediators including cytokines and chemokines, and (3) influences the adaptive immune response. FFA free fatty acids. Adapted and modified from Fitzpatrick’s Dermatology in General Medicine, chapter 22
The Anatomical and Physical Barriers of Innate Immunity
The anatomical barrier of an epidermal surface meets the definition of the innate immune system yet is frequently overlooked by immunologists. In particular, the skin produces sweat and sebum and forms a stratum corneum that guards our inner environment from the outside yet permits and possibly encourages microbial colonization of nonpathogens [2]. A similar phenomenon occurs with mucosal membranes that cover the surface of GI, urogenital, and respiratory tracts and produces saliva, tears, and mucous. Specialized physical elements such as cillia of broncho-pulmonary tree help get rid of microbes where tolerance or the need for symbiotic organisms is less important. Physical factors, such as our normal body temperature, inhibit growth of many microorganisms, whereas the establishment of an acidic pH at the surface provides yet another mechanism to discourage microbial growth. Thus, when considered together, the anatomical/physical barrier is the first innate immune defensive shield. Recognition of this as a part of the immune system as a whole is now leading to greater understanding of how alteration of structural elements may play a role in modifying inflammatory events.
Glycosaminoglycans (GAGs) are a good example of how structural elements are now understood to contribute actively to the immune defense system of the skin. Historically, they were thought to serve as a scaffold necessary for the orientation and organization of the extracellular matrix. However, they are now known to also act as important initiators and regulators of inflammatory responses [3, 4]. In general, GAGs influence cytokine/chemokine production, leukocyte recruitment, and inflammatory cell maturation [5–7]. Among them, hyaluronan (HA) is best known in clinical applications for its role in triggering an inflammatory response. When present in the small molecular weight form, HA is recognized by Toll-like receptor-4 (TLR4) [8, 9] and an inflammatory response is initiated. This system will be discussed further below. Many cells, including keratinocytes, endothelial cells, and fibroblasts, synthesize HA [10–12], and HA production in turn can be induced by proinflammatory cytokines [13]. Among the roles HA may play in an inflammatory response, recruitment of leukocytes to sites of inflammation [13] and regulation of the activation of leukocytes [14] have been suggested. Elevated levels of HA are noted in diseases such as osteoarthritis, rheumatoid arthritis, psoriasis, and scleroderma [10].
The Cellular Components of the Innate Immunity
The cellular compartment of the innate immune system was traditionally believed to consist of only a few cell populations, including neutrophils, macrophages, monocytes, natural killer (NK) cells, and NK T cells (NKT-gamma delta T cells). We now know that innate immunity also involves mast cells that become activated during inflammation. All of these cells rapidly differentiate into short-lived effector cells whose main role is to rid the body of infecting organisms.
Neutrophils (PMN) have classically been known as the most important cells in bacterial destruction. They contain cytoplasmic granules (lysosomes) that carry many secretory components including lysozymes (e.g., myeloperoxidase), proteases (elastase, cathepsin G, etc.), cationic proteins, and defensins. These secretory elements play an integral part in defending our bodies against pathogens. Lysozyme, for example, attacks bacterial peptidoglycan cell walls [15].
Neutrophils, together with macrophages, are major phagocytic cells in the skin. They have the capacity to migrate to the site of infection, detect the pathogen(s) using their receptors [namely, complement receptors and pattern recognition receptors (PRR)] and destroy the invading organisms. In addition to phagocytosis, upon activation, these cells also induce several important effector mechanisms such as triggering the production of cytokines (Fig. 2).
APCs such as macrophages and dendritic cells of the innate immune system survey the environment and recognize pathogens through receptors such as TLRs. Activation of APCs by pathogens leads to phagocytosis, as well as expression of cytokines and costimulatory molecules. On the other hand, APCs present pathogenic antigens (through MHC molecules) and costimulatory molecules to effector cells of the adaptive immune system to direct their response
NK cells, on the other hand, are our major defense against viral infections and malignancies before the adaptive immune responses have had a chance to launch. Simply put, NK cells are lymphoid cells without a specific antigenic receptor that T cells and B cells express. Instead, they recognize the malignant or virus-infected cells via sugar–lectin interaction or become activated in response to interferons (IFNs) or macrophage-derived cytokines [16]. NK cells do not attack the pathogen itself but rather the cells that have been infected by that pathogen. They are considered to be cytotoxic cells due to the possession of small cytoplasmic granules of perforin, granulysin, or other proteases (granzymes) [17, 18]. However, the exact mechanisms of target cell death are not fully known. It has been suggested that perforin forms pores in the cell membrane of the infected cell through which other molecules enter and induce cell apoptosis [19, 20]. Additionally, there are data suggesting that the binding of NK ligands to the target cell’s death receptors such as TRAIL-R [21] induces cell death. Regardless of mechanism of induction, apoptosis is a very effective way to protect the body against viral and malignant spread. In contrast to cell lysis that may lead to the release of pathogens, apoptosis leads to destroying the virus inside the cell.
The Chemical and Secretory Components of the Innate Immunity
The soluble/secretory/chemical group of the innate immune system includes the chemical components that are constitutively produced by a number of cells that prevent pathogen colonization and growth at the interface. Examples of specialized chemical innate barriers include lactic and fatty acids in skin secretions that provide a low pH (5.6–6.4) and the degradation product of sebum-derived triglycerides that contribute to making a hostile environment for pathogen growth. Similarly, the low pH of the stomach and the vagina inhibits microbial growth. Hydrolytic enzymes present in the stomach, such as pepsin, are capable of cleaving proteins.
Following invasion of the epithelial barrier, the complement system, known long before the concepts of innate and adaptive immunity evolved, provides an essential systemic innate chemical defense system. The complement system is best known for induction of phagocytosis (opsonization) and bacterial lysis. Upon activation by a variety of specific and nonspecific immunologic mechanisms, the circulating proteins of the complement system participate in enzymatic cascades resulting in the destruction of pathogenic organisms. Additionally, the complement system is involved in modulating other parts of the immune system such as mast cell activation, neutrophil recruitment, and inflammation [22, 23]. Although the essential cooperativity of the complement cascade is a process that has been rediscovered many times in the innate immune system, the now well described elegant details of this system are beyond the scope of this review. We will just briefly point out that both hereditary deficiencies of complement components (although rare) and acquired deficiencies that result from development of antibodies to complement components, have been reported in association with human diseases. Diseases include but are not limited to autoimmune disorders, recurrent infections (especially meningococcal), and angioedema [24]. In particular, defective regulation of the alternative pathway of complement is associated with autoimmune diseases of the kidney, such as the atypical form of hemolytic uremic syndrome and membranoproliferative glomerulonephritis, and also with age-related macular degeneration of the eye [25].
Other circulating components important to innate immunity were first described in the setting of their activity on cells participating in adaptive responses. This diverse group of regulators, generally referred to as cytokines, includes the growing list of interleukins (ILs), IFNs, chemokines, the tumor necrosis factor (TNF) family, and growth factors. As the list expands (a total of 31 human ILs and over a dozen related cytokines are currently known [26]), the relevance of these proteins to innate or adaptive immunity becomes a subject of frequent reinterpretation.
The diversity of cytokines and the complexity of their interactions make it impossible for us to address this component of the immune system in detail. We will focus on a few cytokines that are most important to our discussion of innate immunity in health and disease. An excellent example of this is interleukin-1 (IL-1). IL-1 is secreted by epithelial cells, macrophages, monocytes, and dendritic cells, and binds the IL-1 receptor (IL-1R), which is similar to the toll-like family of receptors known as classical elements of innate immune pattern recognition [27]. IL-1 and a very similar cytokine, tumor necrosis factor alpha (TNF-α), are classically known for their role in Gram-negative sepsis and endotoxic shock due to bacterial lipopolysaccharide (LPS). In attempts to prevent devastating effects of septic shock, inhibitors of IL-1 and TNF-α such as recombinant human IL-1R antagonist and monoclonal antibody to human TNF-α have been shown to be promising [28, 29]. Recent work with mice deficient in the IL-1R has found that these animals show increased susceptibility to injury [30] and infections (such as gut-derived sepsis caused by Pseudomonas aeruginosa [31] and Listeria monocytogenes infection [30]), a finding consistent with a defective innate immune response or recognition system.
An additional relevant IL worthy of discussion in this overview of the innate immune system is IL-18. IL-18 (IFNγ-inducing factor) has been classified in the IL-1 family based on its structure. However, biological effects of IL-18 are similar to IL-12 in that it mediates a TH1 response and activates NK cells. ILs 12 and 18, like many other cytokines such as IFNs and the TNF family, participate in antimicrobial and antitumor pathways of innate immunity [32, 33]. IL-18 induces IL-1, TNF, and other chemokines [34, 35], whereas IL-12 stimulates the production of IFN gamma, TNF-alpha, as well as the cytotoxic activity of NK cells and CD8+ T lymphocytes [36].
Many studies have identified associations of cytokines with human disease: Increased production of IL-1 and TNF has been linked to Crohn’s disease [37–39]. IL-4 and IL-10 production has been shown in progressive forms of leishmanial, schistosomal, and trypanosomal infections. Additionally, IL-10 expression in lesions correlates with susceptibility to leprosy, which suggests a role for IL-10 in defense against intracellular pathogens [40].
Antimicrobial peptides (AMPs) refer to a group of small peptides, containing less than 100 amino acids, which have an inherent ability to kill a broad range of pathogens, including Gram-negative and Gram-positive bacteria, fungi, and viruses. Their function, once considered to be only antimicrobial, has now been understood to involve many more immunologic activities [41–44]. For example, they act as important regulators by driving cytokine expression in keratinocytes [44, 45]. AMPs are produced in part by the epithelial surfaces as a first-line defense system but also by PMNs, macrophages, platelets, lymphocytes, dendritic cells, and mast cells. Some AMPs are present constitutively although they are not necessarily always active and may require processing [46, 47]. Others are inducible by bacterial products [48, 49], cytokines such as TNF-alpha or IL-1 [41], or vitamin D [50]. AMP production increases with inflammation and injury, and they also have important roles in host repair and adaptive immune response (discussed later).
How Does Innate Immunity Work?
As previously mentioned, innate immunity provides a shield in defense against invading pathogens. As a constitutive system, we have already mentioned several known elements that combine for the basic innate protective system. Skin pH has a key role in fighting bacteria because only few species can proliferate in such low pH [51]. AMPs and proteins can be found at the skin surface and help to hinder microbial proliferation [52, 53]. In fact, some AMPs are produced in greater abundance in the neonatal period [54–56], possibly to compensate for suppressed adaptive immune elements and a more neutral pH [51]. Below this, the stratum corneum barrier consists of several layers of cornified keratinocytes embedded in a lipid matrix that provides a physical barrier. The lipid matrix, composed of ceramides, cholesterol, and free fatty acids, helps form a continuous physical network along the corneocytes and also exerts direct antibacterial activities. Free fatty acids have been described to have antibacterial activity against Staphylococcus aureus [57].
Complementing the constitutive defense system, an inducible system also exists to appropriately respond to danger that is not adequately controlled by the barrier. To simplify our understanding of these elements of the innate immune response system of the skin, we will look at recognition and response systems separately.
Recognition by the Innate Immune System
As discussed earlier, the hallmark of the innate immune response is its speed. As a first step, if a microbe is not controlled by the constitutive barrier and poses a threat to the host, this pathogen must be recognized and discriminated from “self” [58]. The innate immune system plays an important role in discriminating between “infectious nonself” and “noninfectious self” [59]. In addition, we must recognize that a third group, “noninfectious nonself,” must also be distinguished at external epithelial interfaces. The innate immune system of the skin must make the first decision about potential danger and whether or not respond. Most skin disease can be categorized as either inappropriate decision about danger or an inappropriate response to danger.
Pathogen recognition by the innate immune system happens through a limited number of germline-encoded receptors. These receptors are special in that they recognize not one specific but many similar molecules; they are less stringent and more promiscuous receptors than their adaptive counterparts. Therefore, they have been referred to, by some authors, as PRRs. Although we realize that the term “pattern” is not well defined, for simplicity, we will continue to refer to these receptors as PRRs in this discussion. PRRs gain their specificities from inherited genetic material. They have evolved to recognize patterns that are common among pathogens but not present in the host, called pathogen-associated molecular patterns (PAMP). A classic example is the recognition of LPS, a cell wall component of Gram-negative bacteria, by PRRs present on antigen-presenting cells (APCs) and other cells. Macrophages and dendritic cells of the innate immune system continually survey the environment and recognize PAMPs [58, 60]. This is in contrast to the cells of adaptive immune system that can create a large repertoire of specificity by combination of genetic material. This gives the adaptive immune system a much greater variability in recognition potential that is responsive to education. Because these potential lymphocyte receptors are subject to expansion only in response to the exposure of each individual to the target antigen, each generation must reinvent their own repertoire. In contrast, innate immune receptors are limited in number but are retained in evolution and capable of recognizing more broad structural features of a molecule [58].
PRRs of the innate immune system are not confined to the cell membrane. They are also present in intracellular compartments, circulating plasma, and tissues [58, 59, 61]. Once activated, these receptors participate in phagocytosis, opsonization, activation of complement and coagulation cascades, activation of proinflammatory signaling pathways, and induction of apoptosis [62–64].
Among soluble PRRs that bind common structures in microbes are Mannan-binding lectin and ficolins. Upon stimulation, they activate complement factors C2 and C4, leading to activation of the central molecule of the complement cascade, C3. Complement cascade, in turn, promotes opsonization, phagocytosis, and cytokine and chemokine release. The result is a proinflammatory antimicrobial environment that helps eliminate the pathogen [62, 63, 65].
Another important cellular PRR is the macrophage mannose receptor (MMR) of the C-type lectin family. MMR interacts with a variety of Gram-positive and Gram-negative bacteria and fungal pathogens by stimulating their phagocytosis and subsequent delivery into the lysosomal compartment where they are destroyed by lysosomal enzymes [66].
Macrophages also express macrophage scavenger receptor (MSR), a phagocytic PRR, of the scavenger receptor type A (SR-A) family. MSR has a broad specificity, which recognizes double-stranded RNA (dsRNA), LPS, and lipoteichoic acid [67]. MSR protects against endotoxic shock by scavenging LPS [68]. Another SR-A family macrophage receptor is MARCO, which binds to bacterial cell walls and LPS and mediates phagocytosis [69].
The protein kinase receptor (PKR) is a receptor known to become activated after binding to viral dsRNA. Upon activation, PKR phosphorylates and inactivates the translation initiation factor eIF2α, which in turn blocks viral and cellular protein synthesis [70]. PKR activates NF-kB and MAP kinase signaling pathways, leading to the induction of the antiviral type-I IFN genes [71]. To inhibit viral spread, PKR also induces apoptosis of infected cells [71].
Viral dsRNA also binds and activates the 2′-5′-oligoadenylate synthase (OAS)/RNaseL pathway. Activated OAS produces an unusual nucleotide second messenger -2′-5′ oligoadenylate that then activates the RNaseL. In addition to destroying the viral RNA, the RNaseL also cleaves cellular RNAs. This leads to induction of apoptosis in infected cells, which is important as an additional antiviral mechanism [72].
Among the most well known of all PRRs are the toll-like receptors (TLRs) (Fig. 3). They are transmembrane proteins with ligand-binding domains composed of leucine-rich repeats. TLRs are located on the cell surface or in intracellular compartments and play an important role as primary sensors of invading pathogens and initiators of inflammatory and immune responses [65]. There are 10 known human TLRs [60]. They bind certain antigenic ligands of infectious agents and activate distinct signaling pathways resulting in a similar but not identical pattern of antimicrobial mediators. TLRs recognize a diverse set of Pathogen-associated molecular patterns (PAMPs) (Fig. 3). TLR1–6 are expressed on the cell surface and recognize bacterial components; TLR2 associates with TLR1 or TLR6 to recognize lipopeptides from bacteria, peptidoglycan, and lipoteichoic acid; TLR4/CD14 is activated by LPS derived from Gram-negative bacteria; TLR5 recognizes flagellin. On the other hand, the intracellular TLRs bind viral and bacterial components in the cytoplasm; TLR3 recognizes dsRNA that is produced during virus replication, TLR7 and 8 recognize (G + U)-rich-ss RNA, and finally, TLR9 recognizes unmethylated CpG DNA primarily found in bacteria [65].
TLRs mediate innate immune responses in host defense. a After binding of a ligand, TLR couples with adaptor molecule(s) and initiate signaling pathways that lead to the production of inflammatory cytokines and chemokines. In addition, costimulatory molecules are induced that instruct the type of adaptive immune response. b A number of TLRs are expressed on the cell surface and recognize extracellular bacterial components; TLR4 binds LPS, TLR2 binds bacterial peptidoglycan, and TLR5 recognizes flagellin. Intracellular TLRs bind viral and bacterial components including nucleic acids (TLR9), ds RNA (TLR3) or (G + U)-rich-ss RNA (TLR7 and 8). At least four different adaptor molecules are known to associate with TLRs: MyD88, TIR-domain-containing adaptor (TIRAP), TRIF, and TRIF-related adaptor molecule (TRAM)
The mechanisms of downstream signaling and responses of TLR activation are not fully understood. Commonly, TLRs couple with adaptor molecules and initiate signals. At least four different adaptor molecules are known: myeloid differentiation factor 88 (MyD88), TIR-domain-containing adaptor, TIR-domain-containing adaptor protein inducing IFN-beta (TRIF), and TRIF-related adaptor molecule (Fig. 3). Most TLRs activate adaptor molecule MyD88 that in turn activates MAPK-kinases and the transcription factor NF-KB. This leads to expression of several proinflammatory and regulatory cytokines and chemokines, including IL-1 β, TNF-α, IL-6, and IL-12 [65].
Microbial stimulation of TLRs also leads to the activation of signaling pathways that result in production of antimicrobials, triggering of dendritic cell maturation, induction of costimulatory molecules, and increased antigen-presenting capacity. All these activities help to initiate and direct adaptive immune responses. In this way, TLRs have been recognized as the link between innate and adaptive immunity. They have revealed some of the roles of the innate immune system as regulator of adaptive immune response. It is important to emphasize that the regulation of adaptive immunity by TLRs happens at several steps in the process from TLR recognition to the resultant response [58].
On the other hand, the discovery of multiple TLRs and adaptor molecules, in addition to different intracellular signaling pathways associated with each TLR, clearly indicates that the innate immune system has a much higher degree of specificity than previously recognized. Understanding of such concepts as higher specificity of innate immunity and its regulatory role on adaptive immune response necessitates that we look at innate and adaptive immunity as components of one complex and interacting system rather than two separate entities (Fig. 2).
Clinically, the identification and understanding of TLRs and their signaling pathways has brought new hypotheses about the pathophysiology of many diseases and the mechanism of action of some drugs [58]. A greater appreciation of this system has also generated interest in the development of new drugs and therapies.
Disease Associated with Innate Recognition
Acne vulgaris is a disorder of the pilosebaceous unit characterized by inflammatory papules, pustules, and nodules, as well as noninflammatory comedones. An accepted responsible pathogen for acne vulgaris is Propionibacterium acnes, a Gram-positive anaerobe. Propionibacterium acnes antigens presented by APCs induce cytokines that lead to an inflammatory response [73]. New studies have shown that the activation of TLR2 is a critical step in triggering this proinflammatory cytokine response [74]. In other words, production of inflammatory cytokines (IL-6, -8, and -12) in response to P. acnes depends on TLR2. Recent reports also suggest that retinoids used in the treatment of acne vulgaris exert their anti-inflammatory effects by decreasing local expression of TLR2 [75].
There has also been some thought about TLRs being important in the pathogenesis of psoriasis. Baker et al. have suggested that certain microorganisms induce and/or exacerbate disease processes through the activation of TLRs in the skin [76]. They show up-regulation of TLR2 in psoriatic skin; this may reflect the higher presence of Gram-positive bacteria on the keratin layer or, alternatively, results as a secondary effect due to the presence of proinflammatory cytokines and/or altered keratinocyte differentiation in psoriatic plaques [76].
In addition to their role in microbial recognition, there is evolving evidence that TLRs also participate in the recognition of injury by detecting fragmented elements of the host. These host-associated molecular patterns include fragmented hyaluronic acid (HA). As mentioned earlier, detection of low-molecular-weight HA by TLR4 leads to the release of cytokines by dendritic cells [8], epithelial cells [77] and endothelial cells [9], and has been shown in mouse models of lung injury to participate in the development of an inflammatory response [78]. More in-depth understanding of HA and its role in triggering sterile inflammation may shed light on the nature of some inflammatory diseases, such as rheumatoid arthritis and Crohn’s disease, and may lead to better diagnostic and therapeutic strategies.
It is true that, in recent years, TLRs have grabbed a lot of scientific attention and have been studied in detail. Recently, however, two additional families of PRRs have been studied in great detail: the NLRs (NOD-like receptors) [79] and the RLHs (RIG-like helicases) [37]. These receptors are soluble proteins expressed in the cytosol, where they recognize and respond to pathogens that have gained access to the intracellular compartments. The NLRs comprise the NOD and the NALP subgroups [79, 37]. NOD1 and NOD2 of the NOD family detect bacterial peptidoglycan (LPS). They then associate with protein kinase RIP2, which in turn activates the transcription factor NF-κB and the MAP kinase signaling pathways [37, 59, 80–83]. Much of the interest in NOD2 comes from the association of mutations in this gene with Crohn’s disease. Of all the familial Crohn’s disease cases in Western populations, about 50% have been linked to three NOD2 mutants [37, 84, 85]. Studies have also shown evidence that NOD2 participates in innate immunity to Streptococcus pneumoniae [37, 86] and Mycobacteria [37, 87]. Cryopyrin (also called Pypaf1) is a member of the NOD protein family that has been shown to be mutated in several inflammatory diseases, including familial cold urticaria and Muckle–Wells syndrome [88, 89].
Responses of the Innate Immune System
As mentioned above, some responding elements of the innate immune system, such as complement system, are well known. More recently discovered are AMPs. AMPs are regarded as major effectors of microbial killing of innate immunity. We mentioned that AMPs are small peptides with an inherent ability to kill a broad range of pathogens and also trigger host cytokine responses. They are produced by epithelial cells, keratinocytes, and many circulating cells. Of peptides discovered in humans with the ability to kill pathogens, the cathelicidins and defensins are the two main groups that are the most thoroughly studied [90–94]. Human β-defensins are produced, constitutively or as result of inflammatory stimuli, by keratinocytes, as well as epithelia of mucous membranes. Cathelicidin, or LL-37, produced in epithelia and by neutrophils, inhibits the growth of many bacteria and some protozoan parasites, as well as the replication of lentivirus [42, 95–97].
These AMP families have little gene structure in common. However, their physical structures are similar in that they are positively charged and hold their charged residues separately from hydrophobic residues. This structural form enables AMPs to bind to the negatively charged microbial membrane, which is a critical step in their killing effect [97–100]. β-defensins can then directly destroy microorganisms by creating pores in the bacterial cell membrane [41, 101].
AMPs are currently subject to increased scientific attention due to their important immunomodulatory functions that extend beyond killing microbes. They act as important communicators and regulators of both innate and adaptive immune systems by their many roles. Some defensins have been shown to stimulate immature dendritic cells and subsets of T cells [102, 103] and play a chemoattractant role for neutrophils, mast cells, and other immune cells [104–106]. Cathelicidins are also chemoattractants for neutrophils, monocytes, and T lymphocytes [107]. Select AMPs initiate posttranslational processing of IL-1beta [108]. It is also important to note that cells classically considered part of the adaptive immune system contain AMPs and larger antimicrobial proteins that are secreted upon antigenic stimulation. Granulysin is an example of such a molecule and has a wide range of antimicrobial activity [109, 110].
An additional function of interest in studying AMPs is the role they play in wound-healing. AMPs were originally discovered in mammalian skin due to the capacity of one type, the cathelicidin PR-39, to induce changes in proteoglycan synthesis important to the wound repair process [52]. Other cathelicidins, such as the human LL-37, influence keratinocytes’ function during wound repair and accelerate the process. Conversely, growth factors that are produced during wound healing to stimulate the regeneration of damaged tissue, such as insulin-like growth factor-I and TGF-α, which are two growth factors that are important in wound healing, have been reported to induce the expression of the AMPs hCAP-18/LL-37 and human beta-defensin 3 [111]. One could suggest that AMPs play a dual role in wound healing by killing bacteria and also stimulating complex host repair responses [52]. The negative consequences of inappropriate AMP function in wound healing have been confirmed in animal studies showing that inhibiting the activation of cathelicidins in pig wounds leads to greatly increased bacterial colonization [112].
Disease Associated with Innate Immune Responses
Abnormalities in expression or processing of AMPs have been associated with a range of human skin diseases. The cathelicidin LL-37 is elevated in human keratinocytes in lupus erythematosus, contact dermatitis [113], and erythema toxicum neonatorum [56]. AMPs also play an important role in atopic dermatitis (AD). AD patients are particularly susceptible to recurrent skin infections, especially with S. aureus [114]. Altered skin barrier function may partially explain S. aureus colonization in AD. However, considering that barrier defects also exist in psoriasis patients, who are by comparison more resistant to skin infection, necessitated seeking a different explanation for microbial susceptibility of the AD patients [115]. The explanation came with the discovery that psoriatic skin lesions are accompanied with increased AMP production, but AD skin has very low expression of cathelicidins (LL-37) and β-defensins [116, 117].
AMPs may also offer new insights into understanding viral skin infections. Correlations between the cutaneous proliferation of vaccinia virus with the lower expression of cathelicidin in mice [118] support the susceptibility of AD patients to eczema vaccinatum. This serious disorder underlies the contraindication for the use of vaccinia in AD patients as a means for immunization against small pox. Induction of epidermal AMPs has also been shown during the development of verruca vulgaris and condyloma accuminatum [119], and these AMPs can act against human papilloma virus (HPV) infection [120].
Abnormal proteolytic processing of cathelicidin AMPs has recently been associated with the pathogenesis of rosacea [126].
How Separate Really are Innate and Adaptive Immune Systems?
We started our review by mentioning that innate and adaptive immunity were historically considered separate entities. With time, the synergistic and interacting parts of the two systems have become clearer. We are now aware of several mechanisms by which the innate immune system instructs and directs adaptive immune responses [1].
Many molecules first identified for their role in control of adaptive immune cell (T- and B-cell) function in fact act also in the innate response. ILs have traditionally been grouped into families based on their structural similarities or their sharing of ligands and/or receptor subunits. For example IL-12, IL-23, and IL-27 all share structural similarities and some receptor/ligand subunits [26]. On the other hand, they share overlapping, but not completely identical, roles in regulating some immune functions: NK cell activation, T cell proliferation/cytokine production, and antibody class switching. The sharing of the subunits among distinct ILs and/or their ligands/receptors have necessitated the reevaluation of the roles initially ascribed [26]. It is intriguing that the IL-1R shares significant structural similarity with TLRs and a deficiency in IL-1R leads to increased susceptibility to infection by S. aureus in mice [121]. Study of the overlapping roles of such molecules in regulation of innate and adaptive immune systems may clarify other pathways of interaction between the two systems.
Keratinocytes and other epithelia use their innate immune elements to recognize and respond to danger with the production of peptides capable of recruiting leukocytes. APCs influenced by these events further build a bridge to adaptive immunity as they present foreign antigens to T and B lymphocytes, eventually directing a specific response that can be recalled without the initial delay of the naive host (Fig. 2). This way, when the innate immune system is unable to completely control an infection, it will instruct the adaptive immune system about the nature of the pathogenic challenge it is dealing with. After mounting a delaying action, the innate response of the epithelium is joined in its fight by a circulating cellular response using both innate and adaptive elements. Thus, the adaptive immune response learns about danger in the initial setting of innate elements, hopefully gaining the capacity to recall a response to pathogenic nonself while ignoring self and nonpathogenic nonself.
Why Do We Care?
A growing insight into immune defense pathways clearly sheds light on abnormalities associated with human diseases. Identification of novel sensing and signaling mechanisms may help us understand how some of the commonly used drugs work and may also direct our future path in developing new treatments.
The therapeutic potential that understanding innate immunity provides is most dramatically exemplified by the success of several medications recently introduced into clinical practice. Imiquimod (Aldara®), for example, is an immune response modifier of the imidazoquinoline family that is used in dermatology due to its antitumoral and antiviral activity. It has been proven useful as a topical treatment of basal cell carcinomas and cutaneous squamous cell carcinomas in situ, and also in treatment of external genital warts caused by HPV. The study of TLRs gave us insight into how this drug works. Imiquimod binds to TLR7 and TLR8 receptors, which associated with MyD88 receptors, leading to the activation of transcription factor NFκB (Fig. 3). NFκB then enters the nucleus and in turn activates transcription of many inflammatory cytokines and chemokines including TNF-α, IL-2, IL-6, IL-12, G-CSF, GM-CSF, IFN, IFN, and IL-8 [122–125]. The downstream result of Imiquimod binding to TLR receptors is a proinflammatory and cellular immune response directed at the virally infected or tumor cells [122].
In this paper we reviewed some general aspects of the innate immune system and pathways linking innate and adaptive immunity. However, we are just beginning to understand the role of the innate immune system in a variety of clinical diseases and its potential role as treatments. There is much more to learn and we are certainly entering a new era of studying and understanding our immune system both on the bench and at bedside.
References
Clark R, Kupper T (2005) Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol 125:629–637
Roth RR, James WD (1989) Microbiology of the skin: resident flora, ecology, infection. J Am Acad Dermatol 20:367–390
Gotte M, Echtermeyer F (2003) Syndecan-1 as a regulator of chemokine function. ScientificWorldJournal 3:1327–1331
Rose MJ, Page C (2004) Glycosaminoglycans and the regulation of allergic inflammation. Curr Drug Targets Inflamm Allergy 3:221–225
Tkachenko E, Rhodes JM, Simons M (2005) Syndecans: new kids on the signaling block. Circ Res 96:488–500
Trowbridge JM, Gallo RL (2002) Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology 12:117R–125R
Fraser JR, Laurent TC, Laurent UB (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242:27–33
Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC (2002) Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195:99–111
Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279:17079–17084
Laurent TC, Fraser JR (1992) Hyaluronan. FASEB J 6:2397–2404
Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M (1998) Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest 101:97–108
Sayo T, Sugiyama Y, Takahashi Y, Ozawa N, Sakai S, Ishikawa O, Tamura M, Inoue S (2002) Hyaluronan synthase 3 regulates hyaluronan synthesis in cultured human keratinocytes. J Invest Dermatol 118:43–48
Siegelman MH, DeGrendele HC, Estess P (1999) Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol 66:315–321
Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A (2002) Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J Immunol 169:4322–4331
Palaniyar N, Nadesalingam J, Reid KB (2002) Pulmonary innate immune proteins and receptors that interact with gram-positive bacterial ligands. Immunobiology 205:575–594
Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220
Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H (1994) Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31–37
Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A (1998) Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol 161:1758–1764
Liu CC, Walsh CM, Young JD (1995) Perforin: structure and function. Immunol Today 16:194–201
Liu CC, Persechini PM, Young JD (1995) Perforin and lymphocyte-mediated cytolysis. Immunol Rev 146:145–175
Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T, Ogasawara K (2001) Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol 31:3138–3146
Ali H, Panettieri RA Jr (2005) Anaphylatoxin C3a receptors in asthma. Respir Res 6:19
Godau J, Heller T, Hawlisch H, Trappe M, Howells E, Best J, Zwirner J, Verbeek JS, Hogarth PM, Gerard C, Van Rooijen N, Klos A, Gessner JE, Kohl J (2004) C5a initiates the inflammatory cascade in immune complex peritonitis. J Immunol 173:3437–3445
Dragon-Durey MA, Fremeaux-Bacchi V (2006) Complement component deficiencies in human disease. Presse Med 35:861–870
Zipfel PF, Heinen S, Jozsi M, Skerka C (2006) Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol 43:97–106
Beadling C, Slifka MK (2006) Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch Immunol Ther Exp (Warsz) 54:15–24
Gay NJ, Keith FJ (1991) Drosophila Toll and IL-1 receptor. Nature 351:355–356
Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL et al (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271:1836–1843
Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R et al (1995) Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA 273:934–941
Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW (1997) Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol 159:2452–2461
Horino T, Matsumoto T, Uramatsu M, Tanabe M, Tateda K, Miyazaki S, Nakane A, Iwakura Y, Yamaguchi K (2005) Interleukin-1-deficient mice exhibit high sensitivity to gut-derived sepsis caused by Pseudomonas aeruginosa. Cytokine 30:339–346
Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER (2004) Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock 21:415–425
Hikosaka S, Hara I, Miyake H, Hara S, Kamidono S (2004) Antitumor effect of simultaneous transfer of interleukin-12 and interleukin-18 genes and its mechanism in a mouse bladder cancer model. Int J Urol 11:647–652
Dinarello CA (1999) IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol 103:11–24
Laurincova B (2000) Interleukin-1 family: from genes to human disease. Acta Univ Palacki Olomuc Fac Med 143:19–29
Wang KS, Frank DA, Ritz J (2000) Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood 95:3183–3190
Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nature 442:39–44
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
McAlindon ME, Hawkey CJ, Mahida YR (1998) Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut 42:214–219
Yamamura M (1992) Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 255:12
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720
Oppenheim JJ, Biragyn A, Kwak LW, Yang D (2003) Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis 62(Suppl 2):ii17–ii21
Tosi MF (2005) Innate immune responses to infection. J Allergy Clin Immunol 116:241–249 (quiz 250)
Braff MH, Bardan A, Nizet V, Gallo RL (2005) Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol 125:9–13
Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 175:4662–4668
Braff MH, Di Nardo A, Gallo RL (2005) Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol 124:394–400
Menzies BE, Kenoyer A (2006) Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin-3 in skin keratinocytes. Infect Immun 74(12):6847–6854
Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M, Abreu MT (2004) Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 173:5398–5405
Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL (2003) Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 171:6820–6826
Gombart AF, Borregaard N, Koeffler HP (2005) Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077
Mempel M, Schmidt T, Weidinger S, Schnopp C, Foster T, Ring J, Abeck D (1998) Role of Staphylococcus aureus surface-associated proteins in the attachment to cultured HaCaT keratinocytes in a new adhesion assay. J Invest Dermatol 111:452–456
Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M (1994) Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A 91:11035–11039
Harder J, Bartels J, Christophers E, Schroder JM (1997) A peptide antibiotic from human skin. Nature 387:861
Dorschner RA, Lin KH, Murakami M, Gallo RL (2003) Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res 53:566–572
Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, Agerberth B (2003) Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res 53:211–216
Marchini G, Lindow S, Brismar H, Stabi B, Berggren V, Ulfgren AK, Lonne-Rahm S, Agerberth B, Gudmundsson GH (2002) The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol 147:1127–1134
Bibel DJ, Miller SJ, Brown BE, Pandey BB, Elias PM, Shinefield HR, Aly R (1989) Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficient mice. J Invest Dermatol 92:632–638
Kang SS, Kauls LS, Gaspari AA (2006) Toll-like receptors: applications to dermatologic disease. J Am Acad Dermatol 54:951–983 (quiz 983–956)
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Akira S, Hemmi H (2003) Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85:85–95
Medzhitov R, Janeway CA Jr (1997) Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 9:4–9
Matsushita M, Fujita T (1992) Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med 176:1497–1502
Kuhlman M, Joiner K, Ezekowitz RA (1989) The human mannose-binding protein functions as an opsonin. J Exp Med 169:1733–1745
Schweinle JE, Ezekowitz RA, Tenner AJ, Kuhlman M, Joiner KA (1989) Human mannose-binding protein activates the alternative complement pathway and enhances serum bactericidal activity on a mannose-rich isolate of Salmonella. J Clin Invest 84:1821–1829
Hawlisch H, Kohl J (2006) Complement and Toll-like receptors: key regulators of adaptive immune responses. Mol Immunol 43:13–21
Fraser IP, Koziel H, Ezekowitz RA (1998) The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol 10:363–372
Pearson AM (1996) Scavenger receptors in innate immunity. Curr Opin Immunol 8:20–28
Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J (2000) Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med 191:147–156
Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K (1995) Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80:603–609
Clemens MJ, Elia A (1997) The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res 17:503–524
Williams BR (1999) PKR; a sentinel kinase for cellular stress. Oncogene 18:6112–6120
Kumar M, Carmichael GG (1998) Antisense RNA: function and fate of duplex RNA in cells of higher eukaryotes. Microbiol Mol Biol Rev 62:1415–1434
Vowels BR, Yang S, Leyden JJ (1995) Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun 63:3158–3165
Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, Brightbill HD, Holland D, Cunliffe WJ, Akira S, Sieling PA, Godowski PJ, Modlin RL (2002) Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 169:1535–1541
Liu PT, Krutzik SR, Kim J, Modlin RL (2005) Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol 174:2467–2470
Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L (2003) Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 148:670–679
Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, Ouyang B, Garg HG, Hales CA, Quinn DA (2004) Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol 30:51–60
Jiang D, Liang J, Li Y, Noble PW (2006) The role of Toll-like receptors in non-infectious lung injury. Cell Res 16:693–701
Martinon F, Tschopp J (2005) NLRs join TLRs as innate sensors of pathogens. Trends Immunol 26:447–454
Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276:4812–4818
Inohara N, Ogura Y, Chen FF, Muto A, Nunez G (2001) Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 276:2551–2554
Bertin J, Nir WJ, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GH, Glucksmann MA, DiStefano PS (1999) Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J Biol Chem 274:12955–12958
Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Nunez G (1999) Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem 274:14560–14567
Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606
Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S (2004) Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem 279:36426–36432
Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, van Crevel R, Adema GJ, Ottenhoff TH, Van der Meer JW, Netea MG (2005) NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 1:279–285
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat Genet 29:301–305
Dode C, Le Du N, Cuisset L, Letourneur F, Berthelot JM, Vaudour G, Meyrier A, Watts RA, Scott DG, Nicholls A, Granel B, Frances C, Garcier F, Edery P, Boulinguez S, Domergues JP, Delpech M, Grateau G (2002) New mutations of CIAS1 that are responsible for Muckle–Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet 70:1498–1506
Zaiou M, Gallo RL (2002) Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med 80:549–561
Ramanathan B, Davis EG, Ross CR, Blecha F (2002) Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect 4:361–372
Gennaro R, Zanetti M (2000) Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers 55:31–49
Raj PA, Dentino AR (2002) Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett 206:9–18
Cole AM, Ganz T (2000) Human antimicrobial peptides: analysis and application. Biotechniques 29:822–826, 828, 830–831
Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL (2005) Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun 73:6771–6781
Steinstraesser L, Tippler B, Mertens J, Lamme E, Homann HH, Lehnhardt M, Wildner O, Steinau HU, Uberla K (2005) Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology 2:2
Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B (1998) Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem 273:3718–3724
Gutsmann T, Hagge SO, Larrick JW, Seydel U, Wiese A (2001) Interaction of CAP18-derived peptides with membranes made from endotoxins or phospholipids. Biophys J 80:2935–2945
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341(Pt 3):501–513
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim Biophys Acta 1758(9):1184–1202
Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, Lubkowski J (2000) The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem 275:32911–32918
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW (2002) Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298:1025–1029
Yang D, Chertov O, Oppenheim JJ (2001) Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J Leukoc Biol 69:691–697
Hoover DM, Boulegue C, Yang D, Oppenheim JJ, Tucker K, Lu W, Lubkowski J (2002) The structure of human macrophage inflammatory protein-3alpha /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J Biol Chem 277:37647–37654
Niyonsaba F, Ogawa H, Nagaoka I (2004) Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology 111:273–281
Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I (2002) Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol 14:421–426
De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O (2000) LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192:1069–1074
Perregaux DG, Bhavsar K, Contillo L, Shi J, Gabel CA (2002) Antimicrobial peptides initiate IL-1 beta posttranslational processing: a novel role beyond innate immunity. J Immunol 168:3024–3032
Pena SV, Krensky AM (1997) Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol 9:117–125
Krensky AM (2000) Granulysin: a novel antimicrobial peptide of cytolytic T lymphocytes and natural killer cells. Biochem Pharmacol 59:317–320
Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N (2003) Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol 170:5583–5589
Cole AM, Shi J, Ceccarelli A, Kim YH, Park A, Ganz T (2001) Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 97:297–304
Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH (1997) The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 272:15258–15263
Leung DY (2003) Infection in atopic dermatitis. Curr Opin Pediatr 15:399–404
Grice K, Sattar H, Baker H, Sharratt M (1975) The relationship of transepidermal water loss to skin temperature in psoriasis and eczema. J Invest Dermatol 64:313–315
Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347:1151–1160
Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY (2003) Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 171:3262–3269
Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY (2004) Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol 172:1763–1767
Conner K, Nern K, Rudisill J, O’Grady T, Gallo RL (2002) The antimicrobial peptide LL-37 is expressed by keratinocytes in condyloma acuminatum and verruca vulgaris. J Am Acad Dermatol 47:347–350
Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT (2006) Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci U S A 103:1516–1521
Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL (2006) MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24:79–91
Schon MP, Schon M, Klotz KN (2006) The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol 126:1338–1347
Gibson SJ, Imbertson LM, Wagner TL, Testerman TL, Reiter MJ, Miller RL, Tomai MA (1995) Cellular requirements for cytokine production in response to the immunomodulators imiquimod and S-27609. J Interferon Cytokine Res 15:537–545
Reiter MJ, Testerman TL, Miller RL, Weeks CE, Tomai MA (1994) Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol 55:234–240
Wagner TL, Horton VL, Carlson GL, Myhre PE, Gibson SJ, Imbertson LM, Tomai MA (1997) Induction of cytokines in cynomolgus monkeys by the immune response modifiers, imiquimod, S-27609 and S-28463. Cytokine 9:837–845
Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL (2007) Elevated serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nature Medicine 13(8):975–980
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goodarzi, H., Trowbridge, J. & Gallo, R.L. Innate Immunity: A Cutaneous Perspective. Clinic Rev Allerg Immunol 33, 15–26 (2007). https://doi.org/10.1007/s12016-007-0037-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-007-0037-4