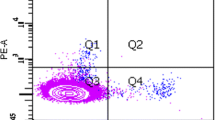
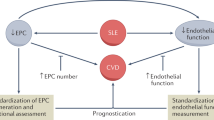
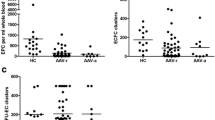
Abstract
Circulating endothelial progenitor cells (EPCs) were first described in 1997 by Asahara et al. as “putative endothelial cells” from human peripheral blood. The study of endothelial progenitors is also intensifying in several pathologies associated with endothelial damage, including diabetes, myocardial infarction, sepsis, pulmonary arterial hypertension, obstructive bronchopneumopathy and transplantation. EPCs have been studied in several autoimmune diseases with endothelial involvement such as systemic lupus erythematosus, thrombotic thrombocytopenic purpura, antineutrophil cytoplasmic antibodies, vasculitis, rheumatoid arthritis, Goujerot-Sjögren and antiphospholipid syndrome. Factors involved in endothelial damage are due to overexpression of pro-inflammatory cytokines and/or autoantibodies. Management of these pathologies, particularly the long-term use of glucocorticoids and methotrexate, promote atherosclerosis. A lack of standardized assessment of the number and function of EPCs represents a serious challenge for the use of EPCs as prognostic markers of cardiovascular diseases (CVD). The objective of this review was to describe EPCs, their properties and their involvement in several autoimmune diseases.
Graphical Abstract



Similar content being viewed by others
References
Alessandri, G., Girelli, M., Taccagni, G., Colombo, A., Nicosia, R., Caruso, A., Baronio, M., Pagano, S., Cova, L., & Parati, E. (2001). Human vasculogenesis ex vivo: Embryonal aorta as a tool for isolation of endothelial cell progenitors. Laboratory Investigation, 81, 875–885.
Rafii, S., Heissig, B., & Hattori, K. (2002). Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Therapy, 9, 631–641.
Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G., & Isner, J. M. (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science, 275, 964–967.
Rossi, E., Poirault-Chassac, S., Bieche, I., Chocron, R., Schnitzler, A., Lokajczyk, A., Bourdoncle, P., Dizier, B., Bacha, N. C., Gendron, N., Blandinieres, A., Guerin, C. L., Gaussem, P., & Smadja, D. M. (2019). Human endothelial colony forming cells express intracellular CD133 that modulates their vasculogenic properties. Stem Cell Review and Reports, 15, 590–600.
Del Díaz, S., Barrena, S., Muñoz-Chápuli, R., & Carmona, R. (2020). Embryonic circulating endothelial progenitor cells. Angiogenesis, 23, 531–541.
Forsythe, J. A., Jiang, B. H., Iyer, N. V., Agani, F., Leung, S. W., Koos, R. D., & Semenza, G. L. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology, 16, 4604–4613.
Ceradini, D. J., Kulkarni, A. R., Callaghan, M. J., Tepper, O. M., Bastidas, N., Kleinman, M. E., Capla, J. M., Galiano, R. D., Levine, J. P., & Gurtner, G. C. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine, 10, 858–864.
Laufs, U., Werner, N., Link, A., Endres, M., Wassmann, S., Jürgens, K., Miche, E., Böhm, M., & Nickenig, G. (2004). Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation, 109, 220–226.
Détriché, G., Gendron, N., Philippe, A., Gruest, M., Billoir, P., Rossi, E., Guerin, C. L., Lokajczyk, A., Brabant, S., Prié, D., Mirault, T., & Smadja, D. M. (2022). Gonadotropins as novel active partners in vascular diseases: Insight from angiogenic properties and thrombotic potential of endothelial colony-forming cells. Journal of Thrombosis and Haemostasis, 20, 230–237.
Urbich, C., & Dimmeler, S. (2004). Endothelial progenitor cells: Characterization and role in vascular biology. Circulation Research, 95, 343–353.
Mak, A., & Chan, J. K. Y. (2022). Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nature Reviews Rheumatology, 18, 286–300.
Medina, R. J., Barber, C. L., Sabatier, F., Dignat-George, F., Melero-Martin, J. M., Khosrotehrani, K., Ohneda, O., Randi, A. M., Chan, J. K. Y., Yamaguchi, T., Van Hinsbergh, V. W. M., Yoder, M. C., & Stitt, A. W. (2017). Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Translational Medicine, 6, 1316–1320.
Smadja, D. M., Melero-Martin, J. M., Eikenboom, J., Bowman, M., Sabatier, F., & Randi, A. M. (2019). Standardization of methods to quantify and culture endothelial colony-forming cells derived from peripheral blood: Position paper from the international society on thrombosis and haemostasis SSC. Journal of Thrombosis and Haemostasis, 17, 1190–1194.
Hill, J. M., Zalos, G., Halcox, J. P. J., Schenke, W. H., Waclawiw, M. A., Quyyumi, A. A., & Finkel, T. (2003). Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England Journal of Medicine, 348, 593–600.
Wils, J., Favre, J., & Bellien, J. (2017). Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacology & Therapeutics, 170, 98–115.
Nuzzolo, E. R., Iachininoto, M. G., & Teofili, L. (2012). Endothelial progenitor cells and thrombosis. Thrombosis Research, 129, 309–313.
Heinisch, P. P., Bello, C., Emmert, M. Y., Carrel, T., Dreßen, M., Hörer, J., Winkler, B., & Luedi, M. M. (2022). Endothelial progenitor cells as biomarkers of cardiovascular pathologies: A narrative review. Cells, 11, 1678.
Altabas, V., Altabas, K., & Kirigin, L. (2016). Endothelial progenitor cells (EPCs) in ageing and age-related diseases: How currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mechanisms of Ageing and Development, 159, 49–62.
Schmidt-Lucke, C., Rössig, L., Fichtlscherer, S., Vasa, M., Britten, M., Kämper, U., Dimmeler, S., & Zeiher, A. M. (2005). Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation, 111, 2981–2987.
Sherer, Y., & Shoenfeld, Y. (2006). Mechanisms of disease: Atherosclerosis in autoimmune diseases. Nature Clinical Practice Rheumatology, 2, 99–106.
Widemann, A., Pasero, C., Arnaud, L., Poullin, P., Loundou, A. D., Choukroun, G., Sanderson, F., Lacroix, R., Sabatier, F., Coppo, P., Dignat-George, F., Kaplanski, G., ENDO-13 study group. (2014). Circulating endothelial cells and progenitors as prognostic factors during autoimmune thrombotic thrombocytopenic purpura: Results of a prospective multicenter french study. Journal of Thrombosis and Haemostasis, 12, 1601–1609.
Bartoloni, E., Alunno, A., Bistoni, O., Caterbi, S., Luccioli, F., Santoboni, G., Mirabelli, G., Cannarile, F., & Gerli, R. (2015). Characterization of circulating endothelial microparticles and endothelial progenitor cells in primary Sjögren’s syndrome: New markers of chronic endothelial damage? Rheumatology (Oxford, England), 54, 536–544.
Alunno, A., Ibba-Manneschi, L., Bistoni, O., Cipriani, S., Topini, F., Gerli, R., & Manetti, M. (2019). Angiogenic T cells in primary Sjögren’s syndrome: A double-edged sword? Clinical and Experimental Rheumatology, 37(Suppl 118), 36–41.
Gresele, P., Migliacci, R., Vedovati, M. C., Ruffatti, A., Becattini, C., Facco, M., Guglielmini, G., Boscaro, E., Mezzasoma, A. M., Momi, S., & Pengo, V. (2009). Patients with primary antiphospholipid antibody syndrome and without associated vascular risk factors present a normal endothelial function. Thrombosis Research, 123, 444–451.
Grenn, R. C., Yalavarthi, S., Gandhi, A. A., Kazzaz, N. M., Núñez-Álvarez, C., Hernández-Ramírez, D., Cabral, A. R., McCune, W. J., Bockenstedt, P. L., & Knight, J. S. (2017). Endothelial progenitor dysfunction associates with a type I interferon signature in primary antiphospholipid syndrome. Annals of the Rheumatic Diseases, 76, 450–457.
Lee, P. Y., Li, Y., Richards, H. B., Chan, F. S., Zhuang, H., Narain, S., Butfiloski, E. J., Sobel, E. S., Reeves, W. H., & Segal, M. S. (2007). Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis and Rheumatism, 56, 3759–3769.
Westerweel, P. E., Luijten, R. K. M. A. C., Hoefer, I. E., Koomans, H. A., Derksen, R. H. W. M., & Verhaar, M. C. (2007). Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Annals of the Rheumatic Diseases, 66, 865–870.
Moonen, J. R. A. J., de Leeuw, K., van Seijen, X. J. G. Y., Kallenberg, C. G. M., van Luyn, M. J. A., Bijl, M., & Harmsen, M. C. (2007). Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Research & Therapy, 9, R84.
Grisar, J., Steiner, C. W., Bonelli, M., Karonitsch, T., Schwarzinger, I., Weigel, G., Steiner, G., & Smolen, J. S. (2008). Systemic lupus erythematosus patients exhibit functional deficiencies of endothelial progenitor cells. Rheumatology (Oxford, England), 47, 1476–1483.
Deng, X. L., Li, X. X., Liu, X. Y., Sun, L., & Liu, R. (2010). Comparative study on circulating endothelial progenitor cells in systemic lupus erythematosus patients at active stage. Rheumatology International, 30, 1429–1436.
Ablin, J. N., Boguslavski, V., Aloush, V., Elkayam, O., Paran, D., Levartovski, D., Caspi, D., & George, J. (2011). Enhanced adhesive properties of endothelial progenitor cells (EPCs) in patients with SLE. Rheumatology International, 31, 773–778.
Baker, J. F., Zhang, L., Imadojemu, S., Sharpe, A., Patil, S., Moore, J. S., Mohler, E. R., & Von Feldt, J. (2012). Circulating endothelial progenitor cells are reduced in SLE in the absence of coronary artery calcification. Rheumatology International, 32, 997–1002.
Mohan, S., Barsalou, J., Bradley, T. J., Slorach, C., Reynolds, J. A., Hasni, S., Thompson, B., Ng, L., Levy, D., Silverman, E., & Kaplan, M. J. (2015). Endothelial progenitor cell phenotype and function are impaired in childhood-onset systemic lupus erythematosus. Arthritis & Rheumatology (Hoboken, NJ), 67, 2257–2262.
Coppolino, G., Campo, S., Bolignano, D., Sturiale, A., Giacobbe, M. S., Loddo, S., & Buemi, M. (2008). Effect of immunoglobulin treatment on endothelial progenitor cells in systemic lupus erythematosus. Annals of the Rheumatic Diseases, 67, 1047–1048.
Oliveira, A. C. D., Arismendi, M. I., Machado, L. S. G., & Sato, E. I. (2022). Ramipril improves endothelial function and increases the number of endothelial progenitor cells in patients with systemic Lupus Erythematosus. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases, 28, 349–353.
Huang, Z., Liu, L., Huang, S., Li, J., Feng, S., Huang, N., Ai, Z., Long, W., & Jiang, L. (2020). Vitamin D (1,25 (OH)2D3) improves endothelial progenitor cells function via enhanced NO secretion in systemic lupus erythematosus. Cardiology Research and Practice, 16(2020), 6802562. https://doi.org/10.1155/2020/6802562
Holmén, C., Elsheikh, E., Stenvinkel, P., Qureshi, A. R., Pettersson, E., Jalkanen, S., & Sumitran-Holgersson, S. (2005). Circulating inflammatory endothelial cells contribute to endothelial progenitor cell dysfunction in patients with vasculitis and kidney involvement. Journal of the American Society of Nephrology, 16, 3110–3120.
Závada, J., Kideryová, L., Pytlík, R., Vanková, Z., & Tesar, V. (2008). Circulating endothelial progenitor cells in patients with ANCA-associated vasculitis. Kidney & Blood Pressure Research, 31, 247–254.
Závada, J., Kideryová, L., Pytlík, R., Hrusková, Z., & Tesar, V. (2009). Reduced number of endothelial progenitor cells is predictive of early relapse in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatology (Oxford, England), 48, 1197–1201.
de Groot, K., Goldberg, C., Bahlmann, F. H., Woywodt, A., Haller, H., Fliser, D., & Haubitz, M. (2007). Vascular endothelial damage and repair in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis and Rheumatism, 56, 3847–3853.
Wilde, B., Mertens, A., Arends, S. J., Rouhl, R. P., Bijleveld, R., Huitema, J., Timmermans, S. A., Damoiseaux, J., Witzke, O., Duijvestijn, A. M., van Paassen, P., van Oostenbrugge, R. J., & Cohen Tervaert, J. W. (2016). Endothelial progenitor cells are differentially impaired in ANCA-associated vasculitis compared to healthy controls. Arthritis Research & Therapy, 18, 147.
Grisar, J., Aletaha, D., Steiner, C. W., Kapral, T., Steiner, S., Seidinger, D., Weigel, G., Schwarzinger, I., Wolozcszuk, W., Steiner, G., & Smolen, J. S. (2005). Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation, 111, 204–211.
Herbrig, K., Haensel, S., Oelschlaegel, U., Pistrosch, F., Foerster, S., & Passauer, J. (2006). Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Annals of the Rheumatic Diseases, 65, 157–163.
Ablin, J. N., Boguslavski, V., Aloush, V., Elkayam, O., Paran, D., Caspi, D., & George, J. (2006). Effect of anti-TNFalpha treatment on circulating endothelial progenitor cells (EPCs) in rheumatoid arthritis. Life Sciences, 79, 2364–2369.
Spinelli, F. R., Metere, A., Barbati, C., Pierdominici, M., Iannuccelli, C., Lucchino, B., Ciciarello, F., Agati, L., Valesini, G., & Di Franco, M. (2013). Effect of therapeutic inhibition of TNF on circulating endothelial progenitor cells in patients with rheumatoid arthritis. Mediators of Inflammation, 2013, 537539.
Grisar, J., Aletaha, D., Steiner, C. W., Kapral, T., Steiner, S., Säemann, M., Schwarzinger, I., Buranyi, B., Steiner, G., & Smolen, J. S. (2007). Endothelial progenitor cells in active rheumatoid arthritis: Effects of tumour necrosis factor and glucocorticoid therapy. Annals of the Rheumatic Diseases, 66, 1284–1288.
Park, Y. J., Kim, J. Y., Park, J., Choi, J. J., Kim, W. U., & Cho, C. S. (2014). Bone erosion is associated with reduction of circulating endothelial progenitor cells and endothelial dysfunction in rheumatoid arthritis. Arthritis and Rheumatology, 66, 1450–1460.
Pulito-Cueto, V., Remuzgo-Martínez, S., Genre, F., Mora-Cuesta, V. M., Iturbe-Fernández, D., Fernández-Rozas, S., Atienza-Mateo, B., Lera-Gómez, L., Alonso-Lecue, P., Rodríguez-Carrio, J., Prieto-Peña, D., Portilla, V., Blanco, R., Corrales, A., Gualillo, O., Cifrián, J. M., López-Mejías, R., & González-Gay, M. A. (2020). Endothelial progenitor cells as a potential biomarker in interstitial lung disease associated with rheumatoid arthritis. Journal of Clinical Medicine, 9, 4098.
Kuwana, M., Okazaki, Y., Yasuoka, H., Kawakami, Y., & Ikeda, Y. (2004). Defective vasculogenesis in systemic sclerosis. Lancet, 364, 603–610.
Del Papa, N., Quirici, N., Soligo, D., Scavullo, C., Cortiana, M., Borsotti, C., Maglione, W., Comina, D. P., Vitali, C., Fraticelli, P., Gabrielli, A., Cortelezzi, A., & Lambertenghi-Deliliers, G. (2006). Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis and Rheumatism, 54, 2605–2615.
Avouac, J., Juin, F., Wipff, J., Couraud, P. O., Chiocchia, G., Kahan, A., Boileau, C., Uzan, G., & Allanore, Y. (2008). Circulating endothelial progenitor cells in systemic sclerosis: Association with disease severity. Annals of the Rheumatic Diseases, 67, 1455–1460.
Allanore, Y., Batteux, F., Avouac, J., Assous, N., Weill, B., & Kahan, A. (2007). Levels of circulating endothelial progenitor cells in systemic sclerosis. Clinical and Experimental Rheumatology, 25, 60–66.
Andrigueti, F. V., Arismendi, M. I., Ebbing, P. C. C., & Kayser, C. (2015). Decreased numbers of endothelial progenitor cells in patients in the early stages of systemic sclerosis. Microvascular Research, 98, 82–87.
Zhu, S., Evans, S., Yan, B., Povsic, T. J., Tapson, V., Goldschmidt-Clermont, P. J., & Dong, C. (2008). Transcriptional regulation of Bim by FOXO3a and akt mediates Scleroderma Serum-Induced apoptosis in endothelial progenitor cells. Circulation, 118, 2156–2165.
Del Papa, N., Quirici, N., Scavullo, C., Gianelli, U., Corti, L., Vitali, C., Ferri, C., Giuggioli, D., Manfredi, A., Maglione, W., Onida, F., Colaci, M., & Bosari, S. (2010). Lambertenghi Deliliers G. Antiendothelial cell antibodies induce apoptosis of bone marrow endothelial progenitors in systemic sclerosis. Journal of Rheumatology, 37, 2053–2063.
Pulito-Cueto, V., Remuzgo-Martínez, S., Genre, F., Atienza-Mateo, B., Mora-Cuesta, V. M., Iturbe-Fernández, D., Lera-Gómez, L., Pérez-Fernández, R., Prieto-Peña, D., Portilla, V., Blanco, R., Corrales, A., Gualillo, O., Cifrián, J. M., López-Mejías, R., & González-Gay, M. A. (2021). Endothelial progenitor cells: Relevant players in the Vasculopathy and Lung Fibrosis Associated with the Presence of interstitial lung disease in systemic sclerosis patients. Biomedicines, 9, 847.
Manetti, M., Pratesi, S., Romano, E., Rosa, I., Bruni, C., Bellando-Randone, S., Guiducci, S., Maggi, E., Ibba-Manneschi, L., & Matucci-Cerinic, M. (2019). Decreased circulating lymphatic endothelial progenitor cells in digital ulcer-complicated systemic sclerosis. Annals of the Rheumatic Diseases, 78, 575–577.
Furuya, Y., Okazaki, Y., Kaji, K., Sato, S., Takehara, K., & Kuwana, M. (2010). Mobilization of endothelial progenitor cells by intravenous cyclophosphamide in patients with systemic sclerosis. Rheumatology (Oxford, England), 49, 2375–2380.
Del Papa, N., Cortiana, M., Vitali, C., Silvestris, I., Maglione, W., Comina, D. P., Lucchi, T., & Cortelezzi, A. (2008). Simvastatin reduces endothelial activation and damage but is partially ineffective in inducing endothelial repair in systemic sclerosis. The Journal of Rheumatology, 35, 1323–1328.
Andrigueti, F. V., Ebbing, P. C. C., Arismendi, M. I., & Kayser, C. (2017). Evaluation of the effect of sildenafil on the microvascular blood flow in patients with systemic sclerosis: A randomised, double-blind, placebo-controlled study. Clinical and Experimental Rheumatology, 35(Suppl 106), 151–158.
Mariette, X., & Criswell, L. A. (2018). Primary Sjögren’s syndrome. New England Journal of Medicine, 378, 931–939.
Mariette, X. (2010). Sjögren’s syndrome pathophysiology. Revue De Medecine Interne, 31(Suppl 1), S2-6.
Gerli, R., Vaudo, G., Bocci, E. B., Schillaci, G., Alunno, A., Luccioli, F., Hijazi, R., Mannarino, E., & Shoenfeld, Y. (2010). Functional impairment of the arterial wall in primary Sjögren’s syndrome: Combined action of immunologic and inflammatory factors. Arthritis Care Res (Hoboken), 62, 712–718.
Vaudo, G., Bocci, E. B., Shoenfeld, Y., Schillaci, G., Wu, R., Del Papa, N., Vitali, C., Delle Monache, F., Marchesi, S., Mannarino, E., & Gerli, R. (2005). Precocious intima-media thickening in patients with primary Sjögren’s syndrome. Arthritis and Rheumatism, 52, 3890–3897.
Pirildar, T., Tikiz, C., Ozkaya, S., Tarhan, S., Utük, O., Tikiz, H., & Tezcan, U. K. (2005). Endothelial dysfunction in patients with primary Sjögren’s syndrome. Rheumatology International, 25, 536–539.
Rachapalli, S. M., Kiely, P. D., & Bourke, B. E. (2009). Prevalence of abnormal ankle brachial index in patients with primary Sjogren’s syndrome. Clinical Rheumatology, 28, 587–590.
Bodolay, E., Koch, A. E., Kim, J., Szegedi, G., & Szekanecz, Z. (2002). Angiogenesis and chemokines in rheumatoid arthritis and other systemic inflammatory rheumatic diseases. Journal of Cellular and Molecular Medicine, 6, 357–376.
Potvin, F., Petitclerc, E., Marceau, F., & Poubelle, P. E. (1997). Mechanisms of action of antimalarials in inflammation: Induction of apoptosis in human endothelial cells. The Journal of Immunology, 158, 1872–1879.
Ghigo, D., Aldieri, E., Todde, R., Costamagna, C., Garbarino, G., Pescarmona, G., & Bosia, A. (1998). Chloroquine stimulates nitric oxide synthesis in murine, porcine, and human endothelial cells. The Journal of Clinical Investigation, 102, 595–605.
Miranda, S., Billoir, P., Damian, L., Thiebaut, P. A., Schapman, D., Le Besnerais, M., Jouen, F., Galas, L., Levesque, H., Le Cam-Duchez, V., Joannides, R., Richard, V., & Benhamou, Y. (2019). Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: Role of reduced inflammation and endothelial dysfunction. PLoS One, 14, e0212614.
Miyakis, S., Lockshin, M. D., Atsumi, T., Branch, D. W., Brey, R. L., Cervera, R., Derksen, R. H. W. M., De Groot, P. G., Koike, T., Meroni, P. L., Reber, G., Shoenfeld, Y., Tincani, A., Vlachoyiannopoulos, P. G., & Krilis, S. A. (2006). International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). Journal of Thrombosis and Haemostasis, 4, 295–306.
Proulle, V., Furie, R. A., Merrill-Skoloff, G., Furie, B. C., & Furie, B. (2014). Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood, 124, 611–622.
Chaturvedi, S., & McCrae, K. R. (2017). Diagnosis and management of the antiphospholipid syndrome. Blood Reviews, 31, 406–417.
Ahmed, K., Vianna, J. L., Khamashta, M. A., & Hughes, G. R. (1992). IL-2, IL-6 and TNF levels in primary antiphospholipid syndrome. Clinical and Experimental Rheumatology, 10, 503.
Benhamou, Y., Bellien, J., Armengol, G., Brakenhielm, E., Adriouch, S., Iacob, M., Remy-Jouet, I., Le Cam-Duchez, V., Monteil, C., Renet, S., Jouen, F., Drouot, L., Menard, J. F., Borg, J. Y., Thuillez, C., Boyer, O., Levesque, H., Richard, V., & Joannidès, R. (2014). Role of toll-like receptors 2 and 4 in mediating endothelial dysfunction and arterial remodeling in primary arterial antiphospholipid syndrome (Vol. 66, pp. 3210–3220). Arthritis and Rheumatology.
Schreiber, K., Sciascia, S., de Groot, P. G., Devreese, K., Jacobsen, S., Ruiz-Irastorza, G., Salmon, J. E., Shoenfeld, Y., Shovman, O., & Hunt, B. J. (2018). Antiphospholipid syndrome. Nature Reviews Disease Primers, 4, 17103.
López-Pedrera, C., Buendía, P., Cuadrado, M. J., Siendones, E., Aguirre, M. A., Barbarroja, N., Montiel-Duarte, C., Torres, A., Khamashta, M., & Velasco, F. (2006). Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-kappaB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis and Rheumatism, 54, 301–311.
Kinev, A. V., & Roubey, R. A. S. (2008). Tissue factor in the antiphospholipid syndrome. Lupus, 17, 952–958.
Pierangeli, S. S., Espinola, R. G., Liu, X., & Harris, E. N. (2001). Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circulation Research, 88, 245–250.
Esdaile, J. M., Abrahamowicz, M., Grodzicky, T., Li, Y., Panaritis, C., du Berger, R., Côte, R., Grover, S. A., Fortin, P. R., Clarke, A. E., & Senécal, J. L. (2001). Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis and Rheumatism, 44, 2331–2337.
Ebner, P., Picard, F., Richter, J., Darrelmann, E., Schneider, M., Strauer, B. E., & Brehm, M. (2010). Accumulation of VEGFR-2+/CD133 + cells and decreased number and impaired functionality of CD34+/VEGFR-2 + cells in patients with SLE. Rheumatology (Oxford, England), 49, 63–72.
Castejon, R., Jimenez-Ortiz, C., Valero-Gonzalez, S., Rosado, S., Mellor, S., & Yebra-Bango, M. (2014). Decreased circulating endothelial progenitor cells as an early risk factor of subclinical atherosclerosis in systemic lupus erythematosus. Rheumatology (Oxford, England), 53, 631–638.
Castejon, R., Jimenez-Ortiz, C., Rosado, S., Tutor-Ureta, P., Mellor-Pita, S., & Yebra-Bango, M. (2016). Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus, 25, 129–136.
Denny, M. F., Thacker, S., Mehta, H., Somers, E. C., Dodick, T., Barrat, F. J., McCune, W. J., & Kaplan, M. J. (2007). Interferon-alpha promotes abnormal vasculogenesis in lupus: A potential pathway for premature atherosclerosis. Blood, 110, 2907–2915.
Ferrante, A., Guggino, G., Di Liberto, D., Ciccia, F., Cipriani, P., Balistreri, C. R., Sireci, G., Giacomelli, R., & Triolo, G. (2016). Endothelial progenitor cells: Are they displaying a function in autoimmune disorders? Mechanisms of Ageing and Development, 159, 44–48.
Lapraik, C., Watts, R., Bacon, P., Carruthers, D., Chakravarty, K., D’Cruz, D., Guillevin, L., Harper, L., Jayne, D., Luqmani, R., Mooney, J., & Scott, D. (2007). BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guidelines for the management of adults with ANCA associated vasculitis. Rheumatology (Oxford, England), 46, 1615–1616.
Halbwachs, L., & Lesavre, P. (2012). Endothelium-neutrophil interactions in ANCA-associated diseases. Journal of the American Society of Nephrology, 23, 1449–1461.
Stassen, P. M., Derks, R. P. H., Kallenberg, C. G. M., & Stegeman, C. A. (2008). Venous thromboembolism in ANCA-associated vasculitis–incidence and risk factors. Rheumatology (Oxford, England), 47, 530–534.
Hong, Y., Eleftheriou, D., Klein, N. J., & Brogan, P. A. (2015). Impaired function of endothelial progenitor cells in children with primary systemic vasculitis. Arthritis Research & Therapy, 17, 292.
Del Rio, A. P. T., Frade-Guanaes, J. O., Ospina-Prieto, S., Duarte, B. K. L., Bertolo, M. B., Ozelo, M. C., & Sachetto, Z. (2022). Impaired repair properties of endothelial colony-forming cells in patients with granulomatosis with polyangiitis. Journal of Cellular and Molecular Medicine, 26, 5044–5053.
Westerweel, P. E., & Verhaar, M. C. (2009). Endothelial progenitor cell dysfunction in rheumatic disease. Nature Reviews Rheumatology, 5, 332–340.
Egan, C. G., Caporali, F., Garcia-Gonzalez, E., Galeazzi, M., & Sorrentino, V. (2008). Endothelial progenitor cells and colony-forming units in rheumatoid arthritis: Association with clinical characteristics. Rheumatology (Oxford, England), 47, 1484–1488.
Adawi, M., Pastukh, N., Saaida, G., Sirchan, R., Watad, A., & Blum, A. (2018). Inhibition of endothelial progenitor cells may explain the high cardiovascular event rate in patients with rheumatoid arthritis. QJM, 111, 525–529.
Yiu, K. H., Wang, S., Mok, M. Y., Ooi, G. C., Khong, P. L., Lau, C. P., Lai, W. H., Wong, L. Y., Lam, K. F., Lau, C. S., & Tse, H. F. (2010). Role of circulating endothelial progenitor cells in patients with rheumatoid arthritis with coronary calcification. Journal of Rheumatology, 37, 529–535.
Rodríguez-Carrio, J., Prado, C., de Paz, B., López, P., Gómez, J., Alperi-López, M., Ballina-García, F. J., & Suárez, A. (2012). Circulating endothelial cells and their progenitors in systemic lupus erythematosus and early rheumatoid arthritis patients. Rheumatology (Oxford, England), 51, 1775–1784.
Rodríguez-Carrio, J., de Paz, B., López, P., Prado, C., Alperi-López, M., Ballina-García, F. J., & Suárez, A. (2014). IFNα serum levels are associated with endothelial progenitor cells imbalance and disease features in rheumatoid arthritis patients. PLoS One, 9, e86069.
Cafaro, G., Petito, E., Bistoni, O., Falcinelli, E., Cipriani, S., Borghi, M. C., Bonifacio, A. F., Giglio, E., Alunno, A., Perricone, C., Gerli, R., Gresele, P., & Bartoloni, E. (2022). Methotrexate improves endothelial function in early rheumatoid arthritis patients after 3 months of treatment. Arthritis Research & Therapy, 24, 236.
Shirinsky, I., Polovnikova, O., Kalinovskaya, N., & Shirinsky, V. (2013). The effects of fenofibrate on inflammation and cardiovascular markers in patients with active rheumatoid arthritis: A pilot study. Rheumatology International, 33, 3045–3048.
Rodríguez-Carrio, J., Alperi-López, M., López, P., Alonso-Castro, S., Ballina-García, F. J., & Suárez, A. (2015). Angiogenic T cells are decreased in rheumatoid arthritis patients. Annals of the Rheumatic Diseases, 74, 921–927.
Denton, C. P., & Khanna, D. (2017). Systemic sclerosis. Lancet, 390, 1685–1699.
Ota, Y., & Kuwana, M. (2020). Endothelial cells and endothelial progenitor cells in the pathogenesis of systemic sclerosis. European Journal of Rheumatology, 7(Suppl 3), S139–S146. https://doi.org/10.5152/eurjrheum.2019.19158
Flavahan, N. A., Flavahan, S., Mitra, S., & Chotani, M. A. (2003). The vasculopathy of Raynaud’s phenomenon and scleroderma. Rheumatic Diseases Clinics of North America, 29, 275–291. vi.
Avouac, J., Meune, C., Ruiz, B., Couraud, P. O., Uzan, G., Boileau, C., Kahan, A., Chiocchia, G., & Allanore, Y. (2012). Angiogenic biomarkers predict the occurrence of digital ulcers in systemic sclerosis. Annals of the Rheumatic Diseases, 71, 394–399.
Avouac, J., Vallucci, M., Smith, V., Senet, P., Ruiz, B., Sulli, A., Pizzorni, C., Frances, C., Chiocchia, G., Cutolo, M., & Allanore, Y. (2013). Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Research & Therapy, 15, R55.
Yamaguchi, Y., Okazaki, Y., Seta, N., Satoh, T., Takahashi, K., Ikezawa, Z., & Kuwana, M. (2010). Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Research & Therapy, 12, R205.
Benyamine, A., Magalon, J., Cointe, S., Lacroix, R., Arnaud, L., Bardin, N., Rossi, P., Francès, Y., Bernard-Guervilly, F., Kaplanski, G., Harlé, J. R., Weiller, P. J., Berbis, P., Braunstein, D., Jouve, E., Lesavre, N., Couranjou, F., Dignat-George, F., Sabatier, F., … Granel, B. (2017). Increased serum levels of fractalkine and mobilisation of CD34 + CD45- endothelial progenitor cells in systemic sclerosis. Arthritis Research & Therapy, 19, 60.
Mok, M. Y., Yiu, K. H., Wong, C. Y., Qiuwaxi, J., Lai, W. H., Wong, W. S., Tse, H. F., & Lau, C. S. (2010). Low circulating level of CD133 + KDR + cells in patients with systemic sclerosis. Clinical and Experimental Rheumatology, 28, S19-25.
Patschan, S., Tampe, D., Müller, C., Seitz, C., Herink, C., Müller, G. A., Zeisberg, E., Zeisberg, M., Henze, E., & Patschan, D. (2016). Early endothelial progenitor cells (eEPCs) in systemic sclerosis (SSc) - dynamics of cellular regeneration and mesenchymal transdifferentiation. Bmc Musculoskeletal Disorders, 17, 339.
Billoir, P., Blandinières, A., Gendron, N., Chocron, R., Gunther, S., Philippe, A., Guerin, C. L., Israël-Biet, D., & Smadja, D. M. (2021). Endothelial colony-forming cells from idiopathic pulmonary fibrosis patients have a high procoagulant potential. Stem Cell Rev Rep, 17, 694–699.
Tinazzi, E., Amelio, E., Marangoni, E., Guerra, C., Puccetti, A., Codella, O. M., Simeoni, S., Cavalieri, E., Montagnana, M., Adani, R., Corrocher, R., & Lunardi, C. (2011). Effects of shock wave therapy in the skin of patients with progressive systemic sclerosis: A pilot study. Rheumatology International, 31, 651–656.
Manetti, M., Pratesi, S., Romano, E., Bellando-Randone, S., Rosa, I., Guiducci, S., Fioretto, B. S., Ibba-Manneschi, L., Maggi, E., & Matucci-Cerinic, M. (2017). Angiogenic T cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis. PLoS One, 12, e0183102.
Acknowledgements
Authors are grateful to Nikki Sabourin-Gibbs, CHU Rouen, for her help in editing the manuscript.
Author information
Authors and Affiliations
Contributions
G Feugray and P Billoir wrote the manuscript, analyzed and interpreted the data. S Miranda, V Lecam Duchez and J Bellien revised the manuscript and results. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Authors state no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feugray, G., Miranda, S., Le Cam Duchez, V. et al. Endothelial Progenitor Cells in Autoimmune Disorders. Stem Cell Rev and Rep 19, 2597–2611 (2023). https://doi.org/10.1007/s12015-023-10617-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10617-y