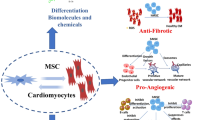
Abstract
Mesenchymal stem cells (MSCs) are so far the most widely researched stem cells in clinics and used as an experimental cellular therapy module, particularly in cardiac regeneration and repair. Ever since the discovery of cardiomyogenesis induction in MSCs, a wide variety of differentiation protocols have been extensively used in preclinical models. However, pre differentiated MSC-derived cardiomyocytes have not been used in clinical trials; highlighting discrepancies and limitations in its use as a source of derived cardiomyocytes for transplantation to improve the damaged heart function. Therefore, this review article focuses on the strategies used to derive cardiomyocytes-like cells from MSCs isolated from three widely used tissue sources and their differentiation efficiencies. We have further discussed the role of MSCs in inducing angiogenesis as a cellular precursor to endothelial cells and its secretory aspects including exosomes. We have then discussed the strategies used for delivering cells in the damaged heart and how its retention plays a critical role in the overall outcome of the therapy. We have also conversed about the scope of the local and systemic modes of delivery of MSCs and the application of biomaterials to improve the overall delivery efficacy and function. We have finally discussed the advantages and limitations of cell delivery to the heart and the future scope of MSCs in cardiac regenerative therapy.
Graphical abstract









Similar content being viewed by others
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- CLC:
-
cardiomyocytes like cells
- CMCs:
-
cardiomyocytes
- iPSC:
-
induced pluripotent stem cells
- ESC:
-
Embryonic Stem Cells
- ADMSC:
-
Adipose-derived Mesenchymal stem cells
- CD:
-
Cluster of Differentiation
- BMSCs:
-
Bone Marrow derived Mesenchymal Stem Cells
- WJ-MSCs:
-
Wharton’s jelly derived Mesenchymal stem cells
- UCB MSC:
-
Umbilical Cord Blood derived Mesenchymal stem cells
- VSELs:
-
Very Small Embryonic Like Stem Cells
- MI:
-
Myocardial Infarction
- ECM:
-
Extracellular Matrix
- 5-Aza:
-
5- Azacytidine
- DMSO:
-
Dimethyl Sulfoxide
- MSC-Exo:
-
MSC derived Exosomes
References
Feric, N.T., Radisic, M. (2016) Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Vol. 96, Advanced Drug Delivery Reviews. p. 110–34.
Benjamin, E.J., Blaha, M.J., Chiuve, S.E., Cushman, M., Das, S.R., Deo, R., et al. (2017) Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Vol. 135, Circulation. p. e146–603.
Roth, G.A., Huffman, M.D., Moran, A.E., Feigin, V., Mensah, G.A., Naghavi, M., et al. (2015) Global and regional patterns in cardiovascular mortality from 1990 to 2013. Vol. 132, Circulation. Lippincott Williams and Wilkins; p. 1667–78.
Vunjak-Novakovic, G., Tandon, N., Godier, A., Maidhof, R., Marsano, A., Martens, T.P., et al. (2010) Challenges in cardiac tissue engineering. Vol. 16, Tissue engineering. Part B, Reviews. p. 169–87.
Cahill, T.J., Choudhury, R.P., Riley, P.R.. (2017) Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Vol. 16, Nature Reviews Drug Discovery. p. 699–717.
Verma, R.S.. (2017) Recent Advances in Induced Pluripotent Stem Cell (iPSC) based Therapeutics. J Stem Cell Res Ther. 3(3).
Cohnheim, J., & Beneke, R. (1894). Ueber Entzündung und Eiterung. Dtsch Medizinische Wochenschrift., 20(12), 276–277.
Friedenstein, A. J., Chailakhjan, R. K., & Lalykina, K. S. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Proliferation, 3(4), 393–403.
Friedenstein, A. J., Chailakhyan, R. K., Latsinik, N. V., Panasyvk, A. F., & Keiliss-Borok, I. V. (1974). Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation., 17(4), 331–340.
Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650.
Makino, S., Fukuda, K., Miyoshi, S., Konishi, F., Kodama, H., Pan, J., Sano, M., Takahashi, T., Hori, S., Abe, H., Hata, J. I., Umezawa, A., & Ogawa, S. (1999). Cardiomyocytes can be generated from marrow stromal cells in vitro. The Journal of Clinical Investigation, 103(5), 697–705.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F. C., Krause, D. S., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy., 8(4), 315–317.
Toma, C., Pittenger, M. F., Cahill, K. S., Byrne, B. J., & Kessler, P. D. (2002 Jan). Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation., 105(1), 93–98.
Guo, Y., Yu, Y., Hu, S., Chen, Y., Shen, Z.. (2020) The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Vol. 11, Cell Death and Disease.
Caplan, A. I. (2017). Mesenchymal stem cells: Time to change the name! Stem Cells Translational Medicine, 6(6), 1445–1451.
Parekkadan, B., Milwid, J.M. (2010) Mesenchymal stem cells as therapeutics. Vol. 12, Annual Review of Biomedical Engineering. p. 87–117.
Levy, O., Kuai, R., Siren, E. M. J., Bhere, D., Milton, Y., Nissar, N., et al. (2020). Shattering barriers toward clinically meaningful MSC therapies. Sci Adv, 6(30), eaba6884.
Kakkar, A., Nandy, S. B., Gupta, S., Bharagava, B., Airan, B., & Mohanty, S. (2019 Oct). Adipose tissue derived mesenchymal stem cells are better respondents to TGFβ1 for in vitro generation of cardiomyocyte-like cells. Molecular and Cellular Biochemistry, 460(1–2), 53–66.
Jin, H. J., Bae, Y. K., Kim, M., Kwon, S. J., Jeon, H. B., Choi, S. J., Kim, S., Yang, Y., Oh, W., & Chang, J. (2013). Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. International Journal of Molecular Sciences, 14(9), 17986–18001.
Rangappa, S., Fen, C., Lee, E. H., Bongso, A., & Wei, E. S. K. (2003). Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. The Annals of Thoracic Surgery, 75(3), 775–779.
Li, Q., Guo, Z. K., Chang, Y. Q., Yu, X., Li, C. X., & Li, H. (2015). Gata4, Tbx5 and Baf60c induce differentiation of adipose tissue-derived mesenchymal stem cells into beating cardiomyocytes. The International Journal of Biochemistry & Cell Biology, 66, 30–36.
Burchfield, J. S., Paul, A. L., Lanka, V., Tan, W., Kong, Y., McCallister, C., Rothermel, B. A., Schneider, J. W., Gillette, T. G., & Hill, J. A. (2016). Pharmacological priming of adipose-derived stem cells promotes myocardial repair. Journal of Investigative Medicine, 64(1), 50–62.
Zhu, Y., Liu, T., Song, K., Ning, R., Ma, X., & Cui, Z. (2009). ADSCs differentiated into cardiomyocytes in cardiac microenvironment. Molecular and Cellular Biochemistry, 324(1–2), 117–129.
Bai, R., Tian, L., Li, Y., Zhang, J., Wei, Y., Jin, Z., Liu, Z., & Liu, H. (2019). Combining ECM hydrogels of cardiac bioactivity with stem cells of high Cardiomyogenic potential for myocardial repair. Stem Cells International, 2019, 1–14.
Ibarra-Ibarra, B., Franco, M., Paez, A., … Cells, E., (2019) U. Improved efficiency of cardiomyocyte-like cell differentiation from rat adipose tissue-derived mesenchymal stem cells with a directed differentiation protocol. hindawi.com.
Schuhheiss, T.M., Burch, J.B.E., Lassar, A.B. (1997) A role for bone morphogenetic proteins in the induction of cardiac myogenesis. genesdev.cshlp.org.
Wijk, B., … (2007) AM-C, 2007 U. Role of bone morphogenetic proteins in cardiac differentiation. academic.oup.com. 74(2):244–55.
Yang, L., Soonpaa, M., Adler, E., (2008) Nature TR-, 2008 U. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. nature.com. 453:524–8.
D’amico, M. A., Ghinassi, B., Izzicupo, P., Di Ruscio, A., & Di Baldassarre, A. (2016 Mar). IL-6 activates PI3K and PKCζ signaling and determines cardiac differentiation in rat embryonic H9c2 cells. Journal of Cellular Physiology, 231(3), 576–586.
Chen, C., Yan, Q., Yan, Y., Ma, M., He, Y., Shui, X., Yang, Z., Lan, X., Tang, Y., & Lei, W. (2018). MicroRNA-1 regulates the differentiation of adipose-derived stem cells into cardiomyocyte-like cells. Stem Cells International, 2018, 1–13.
Zhao, Y., Ransom, J., Li, A., Vedantham, V., & Cell, M. (2007). von D-, 2007 U. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Elsevier., 129(2), 303–317.
Gao, Q., Guo, M., Jiang, X., Hu, X., Wang, Y., & Fan, Y. (2014). A cocktail method for promoting cardiomyocyte differentiation from bone marrow-derived mesenchymal stem cells. Stem Cells International, 2014, 1–11.
Isacchi, B., Fabbri, V., Galeotti, N., Bergonzi, M. C., Karioti, A., Ghelardini, C., Vannucchi, M. G., & Bilia, A. R. (2011). Salvianolic acid B and its liposomal formulations: Anti-hyperalgesic activity in the treatment of neuropathic pain. European Journal of Pharmaceutical Sciences, 44(4), 552–558.
Moghadam, F. H., Tayebi, T., & Barzegar, K. (2016). Differentiation of rat bone marrow mesenchymal stem cells into adipocytes and cardiomyocytes after treatment with platelet lysate. Int J Hematol Stem Cell Res., 10(1), 228–246.
Naaijkens, B. A., Niessen, H. W. M., Prins, H. J., Krijnen, P. A. J., Kokhuis, T. J. A., De Jong, N., et al. (2012 Apr). Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell and Tissue Research, 348(1), 119–130.
Van Den Dolder, J., Mooren, R., Vloon, A. P. G., Stoelinga, P. J. W., & Jansen, J. A. (2006 Nov). Platelet-rich plasma: Quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Engineering, 12(11), 3067–3073.
Filipczyk, A.A., Passier, R., Rochat, A., Mummery, C.L. (2007) Cardiovascular development: Towards biomedical applicability - Regulation of cardiomyocyte differentiation of embryonic stem cells by extracellular signalling. Vol. 64, Cellular and Molecular Life Sciences. p. 704–18.
Hirata, H., Kawamata, S., Murakami, Y., Inoue, K., Nagahashi, A., Tosaka, M., Yoshimura, N., Miyamoto, Y., Iwasaki, H., Asahara, T., & Sawa, Y. (2007). Coexpression of platelet-derived growth factor receptor alpha and fetal liver kinase 1 enhances cardiogenic potential in embryonic stem cell differentiation in vitro. Journal of Bioscience and Bioengineering, 103(5), 412–419.
Lu, G., Haider, H. K., Jiang, S., & Ashraf, M. (2009). Sca-1 + stem cell survival and engraftment in the infarcted heart: Dual role for preconditioning-induced connexin-43. Circulation., 119(19), 2587–2596.
Cao, C., Li, L., Li, H., He, X., Wu, G., & Yu, X. (2018). Cyclic biaxial tensile strain promotes bone marrow-derived mesenchymal stem cells to differentiate into cardiomyocyte-like cells by miRNA-27a. The International Journal of Biochemistry & Cell Biology, 99, 125–132.
Zuo, B., Zhu, J. F., Li, J., Wang, C. D., Zhao, X. Y., Cai, G. Q., Li, Z., Peng, J., Wang, P., Shen, C., Huang, Y., Xu, J., Zhang, X. L., & Chen, X. D. (2015 Feb). microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. Journal of Bone and Mineral Research, 30(2), 330–345.
Yang, S., Shuai, L. W., Dong, F., Mei, S. H., Wu, B., Tan, J., et al. (2014 Oct). KITLG is a novel target of miR-34c that is associated with the inhibition of growth and invasion in colorectal cancer cells. Journal of Cellular and Molecular Medicine, 18(10), 2092–2102.
He, X., Li, L., Tang, M., Zeng, Y., Li, H., & Yu, X. (2019). Biomimetic electrical stimulation induces rat bone marrow mesenchymal stem cells to differentiate into cardiomyocyte-like cells via TGF-beta 1 in vitro. Progress in Biophysics and Molecular Biology, 148, 47–53.
Santhakumar, R., Vidyasekar, P., Verma, R.S. (2014) Cardiogel: A nano-matrix scaffold with potential application in cardiac regeneration using mesenchymal stem cells. PLoS One.;9(12).
Sreejit, P., & Verma, R. S. (2011). Cardiogel supports adhesion, proliferation and differentiation of stem cells with increased oxidative stress protection. Eur Cells Mater., 21, 107–121.
Sreejit, P., Verma, R.S. (2013) Natural ECM as Biomaterial for Scaffold Based Cardiac Regeneration Using Adult Bone Marrow Derived Stem Cells. Vol. 9, Stem Cell Reviews and Reports. p. 158–71.
Sreejit, P., & Verma, R. S. (2013 Sep). Enhanced cardiomyogenic lineage differentiation of adult bone-marrow-derived stem cells grown on cardiogel. Cell and Tissue Research, 353(3), 443–456.
Joshi, J., Brennan, D., Beachley, V., & Kothapalli, C. R. (2018 Dec). Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nanofiber mats. J Biomed Mater Res - Part A., 106(12), 3303–3312.
Dong, C., Lv, Y. (2016) Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Vol. 8, Polymers.
Cao, Y., Xiong, J., Mei, S., Wang, F., Zhao, Z., Wang, S., et al. (2015) Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther 6(1).
Hao, W., Shi, S., Zhou, S., Wang, X., & Nie, S. (2018). Aspirin inhibits growth and enhances cardiomyocyte differentiation of bone marrow mesenchymal stem cells. European Journal of Pharmacology, 827, 198–207.
Nagamura-Inoue, T. (2014). Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells., 6(2), 195–202.
Govarthanan, K., Gupta, P. K., Ramasamy, D., Kumar, P., Mahadevan, S., & Verma, R. S. (2020). DNA methylation microarray uncovers a permissive methylome for cardiomyocyte differentiation in human mesenchymal stem cells. Genomics., 112(2), 1384–1395.
Govarthanan, K., Vidyasekar, P., Gupta, P. K., Lenka, N., & Verma, R. S. (2020). Glycogen synthase kinase 3β inhibitor- CHIR 99021 augments the differentiation potential of mesenchymal stem cells. Cytotherapy., 22(2), 91–105.
Rabbani, S., Soleimani, M., Imani, M., Sahebjam, M., Ghiaseddin, A., Nassiri, S. M., Majd Ardakani, J., Tajik Rostami, M., Jalali, A., Mousanassab, B., Kheradmandi, M., & Ahmadi Tafti, S. H. (2017 Apr). Regenerating heart using a novel compound and human Wharton jelly Mesenchymal stem cells. Archives of Medical Research, 48(3), 228–237.
Bonafè, F., Govoni, M., Giordano, E., Caldarera, C.M., Guarnieri, C., Muscari, C. (2014) Hyaluronan and cardiac regeneration. Vol. 21, Journal of Biomedical Science. BioMed Central Ltd.
Motlagh, D., Senyo, S. E., Desai, T. A., & Russell, B. (2003). Microtextured substrata alter gene expression, protein localization and the shape of cardiac myocytes. Biomaterials., 24(14), 2463–2476.
Jongpaiboonkit, L., King, W. J., Lyons, G. E., Paguirigan, A. L., Warrick, J. W., Beebe, D. J., & Murphy, W. L. (2008). An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials., 29(23), 3346–3356.
Wang, T., Jiang, X. J., Tang, Q. Z., Li, X. Y., Lin, T., Wu, D. Q., Zhang, X. Z., & Okello, E. (2009). Bone marrow stem cells implantation with α-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomaterialia, 5(8), 2939–2944.
Pham, T. L. B., Nguyen, T. T., Van Bui, A., Nguyen, M. T., & Van Pham, P. (2016 Aug). Fetal heart extract facilitates the differentiation of human umbilical cord blood-derived mesenchymal stem cells into heart muscle precursor cells. Cytotechnology., 68(4), 645–658.
Nishiyama, N., Miyoshi, S., Hida, N., Uyama, T., Okamoto, K., Ikegami, Y., Miyado, K., Segawa, K., Terai, M., Sakamoto, M., Ogawa, S., & Umezawa, A. (2007 Aug). The significant Cardiomyogenic potential of human umbilical cord blood-derived Mesenchymal stem cells in vitro. Stem Cells, 25(8), 2017–2024.
Siegel, G., Krause, P., Wöhrle, S., Nowak, P., Ayturan, M., Kluba, T., Brehm, B. R., Neumeister, B., Köhler, D., Rosenberger, P., Just, L., Northoff, H., & Schäfer, R. (2012 Sep). Bone marrow-derived human mesenchymal stem cells express cardiomyogenic proteins but do not exhibit functional cardiomyogenic differentiation potential. Stem Cells and Development, 21(13), 2457–2470.
Liau, B., Zhang, D., Bursac, N. (2012) Functional cardiac tissue engineering. Vol. 7, Regenerative Medicine. p. 187–206.
Gillum, N., & Toxicology, N. S.-C. (2008). 2008 U. adhesion proteins, stem cells, and arrhythmogenesis. Springer., 8, 1–13.
Kim, Y. S., Ahn, Y., Kwon, J. S., Cho, Y. K., Jeong, M. H., Cho, J. G., Park, J. C., & Kang, J. C. (2012). Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells, Tissues, Organs, 195(5), 428–442.
Hatami, L., Valojerdi, M. R., & Mowla, S. J. (2007). Effects of oxytocin on cardiomyocyte differentiation from mouse embryonic stem cells. International Journal of Cardiology, 117(1), 80–89.
Matsuura, K., Nagai, T., Nishigaki, N., Oyama, T., Nishi, J., Wada, H., Sano, M., Toko, H., Akazawa, H., Sato, T., Nakaya, H., Kasanuki, H., & Komuro, I. (2004). Adult cardiac Sca-1-positive cells differentiate into beating Cardiomyocytes. The Journal of Biological Chemistry, 279(12), 11384–11391.
Ratajczak, M. Z., Zuba-Surma, E., Wojakowski, W., Suszynska, M., Mierzejewska, K., Liu, R., Ratajczak, J., Shin, D. M., & Kucia, M. (2014). Very small embryonic-like stem cells (VSELs) represent a real challenge in stem cell biology: Recent pros and cons in the midst of a lively debate. Leukemia., 28, 473–484.
Zuba-Surma, E. K., Wojakowski, W., Ratajczak, M. Z., & Dawn, B. (2011). Very small embryonic-like stem cells: Biology and therapeutic potential for heart repair. Antioxidants and Redox Signaling., 15, 1821–1834.
Hénon, P. (2020). Key success factors for regenerative medicine in acquired heart diseases. Stem Cell Reviews and Reports., 16, 441–458.
Sun, X. L., Li, H. X., Zhu, Y., Xu, P., Zuo, Q. S., Li, B. C., & Gu, X. (2020). 5-Azacytidine-induced Cardiomyocyte differentiation of very small embryonic-like stem cells. Stem Cells International, 2020, 1–8.
Mayourian, J., Cashman, T.J., Ceholski, D.K., Johnson, B.V., Sachs, D., Kaji, D.A., et al. (2017) Experimental and Computational Insight into Human Mesenchymal Stem Cell Paracrine Signaling and Heterocellular Coupling Effects on Cardiac Contractility and Arrhythmogenicity. Circ Res.
Sun, Q., Zhang, Z., Sun, Z. (2014) The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes and Diseases
Niwa, H. (2007) How is pluripotency determined and maintained? Development.
Zomer, H.D., Vidane, A.S., Gonçalves, N.N., Ambrósio, C.E.. (2015) Mesenchymal and induced pluripotent stem cells: General insights and clinical perspectives. Stem Cells and Cloning: Advances and Applications.
Guo, X., Bai, Y., Zhang, L., Zhang, B., Zagidullin, N., Carvalho, K., et al. (2018) Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Vol. 9, Stem Cell Research and Therapy. BioMed Central Ltd.
Moens, S., Goveia, J., Stapor, P.C., Cantelmo, A.R., Carmeliet, P. (2014) The multifaceted activity of VEGF in angiogenesis - Implications for therapy responses. Cytokine and Growth Factor Reviews.
Abhinand, C. S., Raju, R., Soumya, S. J., Arya, P. S., & Sudhakaran, P. R. (2016). VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal., 10, 347–354.
Koch, S., Tugues, S., Li, X., Gualandi, L., Claesson-welsh, L.. (2011) Signal transduction by vascular endothelial growth factor receptors. Biochemical Journal.
Zhou, M., Liu, Z., Liu, C., Jiang, X., Wei, Z., Qiao, W., Ran, F., Wang, W., Qiao, T., & Liu, C. (2012). Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J Biomed Mater Res - Part B Appl Biomater., 100B, 111–120.
Petit, I., Jin, D., & Rafii, S. (2007). The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends in Immunology, 28, 299–307.
Kaga, T., Kawano, H., Sakaguchi, M., Nakazawa, T., Taniyama, Y., & Morishita, R. (2012). Hepatocyte growth factor stimulated angiogenesis without inflammation: Differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascular Pharmacology, 57, 3–9.
You, W.K., McDonald, D.M. (2008) The hepatocyte growth factor/c-met signaling pathway as a therapeutic target to inhibit angiogenesis. Journal of Biochemistry and Molecular Biology.
Kwon, H.M., Hur, S.M., Park, K.Y., Kim, C.K., Kim, Y.M., Kim, H.S., et al. (2014) Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol.
Battegay, E.J., Rupp, J., Iruela-Arispe, L., Sage, E.H., Pech, M. (1994) PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF β-receptors. J Cell Biol.
Klein, D., Weißhardt, P., Kleff, V., Jastrow, H., Jakob, H.G., Ergün, S. (2011) Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One. 6(5).
Wang, H. H., Meng, M. B., Wu, Z. Q., Guo, W. H., Jiang, B., Ying, G. G., Zhao, L. J., Yuan, Z. Y., & Wang, P. (2015). Mesenchymal stem cells generate Pericytes to promote tumor recurrence via Vasculogenesis after stereotactic body radiation therapy. Int J Radiat Oncol., 93(3), E532.
Loibl, M., Binder, A., Herrmann, M., Duttenhoefer, F., Richards, R. G., Nerlich, M., Alini, M., & Verrier, S. (2014). Direct cell-cell contact between mesenchymal stem cells and endothelial progenitor cells induces a pericyte-like phenotype in vitro. BioMed Research International, 2014, 1–10.
Lepidi, S.. (2018) Commentary on “Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in vitro.” Vol. 55, European Journal of Vascular and Endovascular Surgery. p. 266.
Khaki, M., Salmanian, A.H., Abtahi, H., Ganji, A., Mosayebi, G. (2018) Mesenchymal stem cells differentiate to endothelial cells using recombinant vascular endothelial growth factor -A. Reports Biochem Mol Biol.
Ji, S. T., Kim, H., Yun, J., Chung, J. S., & Kwon, S. M. (2017). Promising therapeutic strategies for Mesenchymal stem Cell-based cardiovascular regeneration: From Cell priming to tissue engineering. Stem Cells International, 2017, 1–13.
Shi, S., Sun, J., Meng, Q., Yu, Y., Huang, H., Ma, T., Yang, Z., Liu, X., Yang, J., & Shen, Z. (2018). Sonic hedgehog promotes endothelial differentiation of bone marrow mesenchymal stem cells via VEGF-D. Journal of Thoracic Disease, 10(9), 5476–5488.
Tancharoen, W., Aungsuchawan, S., Pothacharoen, P., Bumroongkit, K., Puaninta, C., Pangjaidee, N., et al. (2019) Human platelet lysate as an alternative to fetal bovine serum for culture and endothelial differentiation of human amniotic fluid mesenchymal stem cells. Mol Med Rep.
Dan, P., Velot, É., Decot, V., & Menu, P. (2015). The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. Journal of Cell Science, 128, 2415–2422.
Gnecchi, M., He, H., Liang, O. D., Melo, L. G., Morello, F., Mu, H., Noiseux, N., Zhang, L., Pratt, R. E., Ingwall, J. S., & Dzau, V. J. (2005). Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells [2]. Nature Medicine., 11, 367–368.
Wang, J., Bonacquisti, E.E., Brown, A.D., Nguyen, J.. (2020) Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells.
Yin, K., Wang, S., & Zhao, R. C. (2019). Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomarker Research, 7, 8.
Lai, R.C., Yeo, R.W.Y., Tan, K.H., Lim, S.K.. (2013) Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regenerative Medicine.
Yu, B., Gong, M., Wang, Y., Millard, R.W., Pasha, Z., Yang, Y., et al. (2013) Cardiomyocyte protection by GATA-4 gene engineered Mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One.
Li, X., Arslan, F., Ren, Y., Adav, S.S., Poh, K.K., Sorokin, V., et al. (2012) Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned by temporal and quantitative changes in the cardiac proteome. J Proteome Res
Azevedo, P.S., Polegato, B.F., Minicucci, M.F., Paiva, S.A.R., Zornoff, L.A.M. (2016) Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arquivos brasileiros de cardiologia.
Vrijsen, K.R., Maring, J.A., Chamuleau, S.A.J., Verhage, V., Mol, E.A., Deddens, J.C., et al. (2016) Exosomes from Cardiomyocyte Progenitor Cells and Mesenchymal Stem Cells Stimulate Angiogenesis Via EMMPRIN. Adv Healthc Mater.
Tongers, J., Losordo, D.W., Landmesser, U.. (2011) Stem and progenitor cell-based therapy in ischaemic heart disease: Promise, uncertainties, and challenges. Vol. 32, European Heart Journal. p. 1197–206.
Psaltis, P., Schwarz, N., Toledo-Flores, D., & Nicholls, J. S. (2016). Cellular therapy for heart failure. Current Cardiology Reviews, 12(3), 195–215.
Mahmoudi, M., Yu, M., Serpooshan, V., Wu, J.C., Langer, R., Lee, R.T., et al. (2017) Multiscale technologies for treatment of ischemic cardiomyopathy. Vol. 12, Nature Nanotechnology. p. 845–55.
Hou, D.., Youssef, E.A.S., Brinton, T.J., Zhang, P., Rogers, P., Price, E.T., et al. (2005) Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 112(9 SUPPL.).
Mitchell, A. J., Sabondjian, E., Sykes, J., Deans, L., Zhu, W., Lu, X., Feng, Q., Prato, F. S., & Wisenberg, G. (2010). Comparison of initial cell retention and clearance kinetics after subendocardial or subepicardial injections of endothelial progenitor cells in a canine myocardial infarction model. Journal of Nuclear Medicine, 51(3), 413–417.
Liu, Z., Mikrani, R., Zubair, H.M., Taleb, A., Naveed, M., Baig, M.M.F.A., et al. (2020) Systemic and local delivery of mesenchymal stem cells for heart renovation: Challenges and innovations. European Journal of Pharmacology.
Wang, W., Jiang, Q, Zhang, H., Jin, P., Yuan, X., Wei, Y., et al. (2011) Intravenous administration of bone marrow mesenchymal stromal cells is safe for the lung in a chronic myocardial infarction model. Regen Med.
Mokhtari, B., Aboutaleb, N., Nazarinia, D., Nikougoftar, M., Razavi Tousi, S., Molazem, M., et al. (2020). Comparison of the effects of intramyocardial and intravenous injections of human mesenchymal stem cells on cardiac regeneration after heart failure. Iranian Journal of Basic Medical Sciences.
Luger, D., Lipinski, M.J., Westman, P.C., Glover, D.K., Dimastromatteo, J., Frias, J.C., et al. (2017) Intravenously delivered mesenchymal stem cells. Circ Res.
Xie, D. M., Li, Y. L., Li, J., Li, Q., Lu, G., Zhai, Y., Zhang, J., Huang, Z., & Gao, X. (2019). CD51 distinguishes a subpopulation of bone marrow mesenchymal stem cells with distinct migratory potential: A novel cell-based strategy to treat acute myocardial infarction in mice. Stem Cell Research & Therapy, 10, 331.
Assis ACM, Carvalho JL, Jacoby BA, Ferreira RLB, Castanheira P, Diniz, SOF, et al. (2010) Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant.
Walczak, P., Zhang, J., Gilad, A. A., Kedziorek, D. A., Ruiz-Cabello, J., Young, R. G., Pittenger, M. F., van Zijl, P. C. M., Huang, J., & Bulte, J. W. M. (2008 May). Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke., 39(5), 1569–1574.
Ottersbach, A., Mykhaylyk, O., Heidsieck, A., Eberbeck, D., Rieck, S., Zimmermann, K., Breitbach, M., Engelbrecht, B., Brügmann, T., Hesse, M., Welz, A., Sasse, P., Wenzel, D., Plank, C., Gleich, B., Hölzel, M., Bloch, W., Pfeifer, A., Fleischmann, B. K., & Roell, W. (2018). Improved heart repair upon myocardial infarction: Combination of magnetic nanoparticles and tailored magnets strongly increases engraftment of myocytes. Biomaterials., 155, 176–190.
Sepantafar, M., Maheronnaghsh, R., Mohammadi, H., Rajabi-Zeleti, S., Annabi, N., Aghdami, N., et al. (2016) Stem cells and injectable hydrogels: Synergistic therapeutics in myocardial repair. Vol. 34, Biotechnology Advances. p. 362–79.
Reis, L. A., Chiu, L. L. Y., Feric, N., Fu, L., & Radisic, M. (2016). Biomaterials in myocardial tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 10(1), 11–28.
Kapoor, S., Kundu, S.C.. (2016) Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Vol. 31, Acta Biomaterialia. p. 17–32.
Ren, S., Jiang, X., Li, Z., Wen, Y., Chen, D., Li, X., Zhang, X., Zhuo, R., & Chu, H. (2012). Physical properties of poly (N-isopropylacrylamide) hydrogel promote its effects on cardiac protection after myocardial infarction. The Journal of International Medical Research, 40(6), 2167–2182.
Nelson, D.M., Ma, Z., Fujimoto, K.L., Hashizume, R., Wagner, W.R.. (2011) Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Vol. 7, Acta Biomaterialia. p. 1–15.
Gupta, S., Sharma, A., & Verma, R. S. (2020). Polymers in biosensor devices for cardiovascular applications. Current Opinion in Biomedical Engineering., 13, 69–75.
Perea-Gil, I., Prat-Vidal, C., Bayes-Genis, A.. (2015) In vivo experience with natural scaffolds for myocardial infarction: The times they are a-changin’. Vol. 6, Stem Cell Research and Therapy. BioMed Central.
Mukherjee, R., Zavadzkas, J. A., Saunders, S. M., McLean, J. E., Jeffords, L. B., Beck, C., et al. (2008). Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. The Annals of Thoracic Surgery, 86(4), 1268–1276.
Wang, H., Shi, J., Wang, Y., Yin, Y., Wang, L., Liu, J., Liu, Z., Duan, C., Zhu, P., & Wang, C. (2014). Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials., 35(13), 3986–3998.
Neshati, V., Mollazadeh, S., Fazly Bazzaz, B. S., de Vries, A. A. F., Mojarrad, M., Naderi-Meshkin, H., Neshati, Z., & Kerachian, M. A. (2018 Jul). Cardiomyogenic differentiation of human adipose-derived mesenchymal stem cells transduced with Tbx20-encoding lentiviral vectors. Journal of Cellular Biochemistry, 119(7), 6146–6153.
Zhang, F., Xie, Y., Bian, Y. (2018) DMPE-PEG scaffold binding with TGF-β1 receptor enhances cardiomyogenic differentiation of adipose-derived stem cells. Stem Cell Res Ther.;9(1).
Wang Y Li, Zhang G, Wang H jie, Tan Y zhen, Wang X yan. Preinduction with bone morphogenetic protein-2 enhances cardiomyogenic differentiation of c-kit+ mesenchymal stem cells and repair of infarcted myocardium. Int J Cardiol. 2018;265:173–80.
Lv, Y., Liu, B., Wang, H. P., & Zhang, L. (2016). Intramyocardial implantation of differentiated rat bone marrow mesenchymal stem cells enhanced by TGF-β1 improves cardiac function in heart failure rats. Brazilian J Med Biol Res = Rev Bras Pesqui medicas e Biol, 49(6), e5273.
Ruan, Z. B., Zhu, L., Yin, Y. G., & Chen, G. C. (2016 Aug). Inhibitor of p53–p21 pathway induces the differentiation of human umbilical cord derived mesenchymal stem cells into cardiomyogenic cells. Cytotechnology., 68(4), 1257–1265.
Ali, S. R., Ahmad, W., Naeem, N., Salim, A., & Khan, I. (2020). Small molecule 2′-deoxycytidine differentiates human umbilical cord-derived MSCs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. Molecular and Cellular Biochemistry, 470(1–2), 99–113.
Le-Buu Pham, T., Nguyen, T.T., Thi-Van Bui, A., Pham, H.T., Phan, N.K., Thi-Thu Nguyen, M., et al. (2015) Preliminary evaluation of treatment efficacy of umbilical cord blood-derived mesenchymal stem cell-differentiated cardiac progenitor cells in a myocardial injury mouse model. Biomed Res Ther. 2(12).
Pereira, W. C., Khushnooma, I., Madkaikar, M., & Ghosh, K. (2008). Reproducible methodology for the isolation of mesenchymal stem cells from human umbilical cord and its potential for cardiomyocyte generation. Journal of Tissue Engineering and Regenerative Medicine, 2(7), 394–399.
Ruan, Z., Zhu, L., Yin, Y., & Chen, G. (2016). Overexpressing NKx2.5 increases the differentiation of human umbilical cord drived mesenchymal stem cells into cardiomyocyte-like cells. Biomed Pharmacother, 78, 110–115.
Acknowledgements
SG would like to acknowledge ICMR for fellowship (3/1/3/JRF-2015/HRD-LS/90/40282/91). The authors would also like to acknowledge the support by the Department of Biotechnology (DBT), India for their funding and support (BT/PR8587/MED/31/236/2013).
Availability of Data and Materials
Not applicable.
Funding
Indian Council of Medical Research (ICMR) and the Department of Biotechnology (DBT), India.
Author information
Authors and Affiliations
Contributions
SG - Conceptualization, Literature Study, Manuscript preparation; AS - Literature Study, Manuscript preparation; AJ - Literature Study, Manuscript preparation; RSV - Conceptualization, Literature Study, Manuscript preparation.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declares that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, S., Sharma, A., S, A. et al. Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery. Stem Cell Rev and Rep 17, 1666–1694 (2021). https://doi.org/10.1007/s12015-021-10168-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10168-0