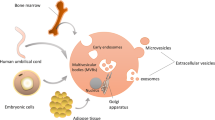
Abstract
Bone marrow mesenchymal stem cells have been investigated for many years, especially for tissue regeneration, and have inherent limitations. One of the rapidly developing fields in the scientific world in recent years is extracellular vesicles. Especially, bone marrow mesenchymal stem cell originated extracellular vesicles are known to have positive contributions in tissue regeneration, and these extracellular vesicles have also been used as gene transfer systems for cellular therapy. Through gene expression analysis and bioinformatics tools, it is possible to determine which genes have changed in the targeted tissue or cell and which miRNAs that can correct this gene expression disorder. This approach connecting the stem cell, extracellular vesicles, epigenetics regulation and bioinformatics fields is one of the promising areas for the treatment of diseases in the future. With this review, it is aimed to present the studies carried out for the use of bone marrow stem cell-derived extracellular vesicles loaded with targeted miRNAs in different in vivo and in vitro human disease models and to discuss recent developments in this field.

Similar content being viewed by others
Abbreviations
- 3’ UTR :
-
3′-Untranslated Region
- ADAM9 :
-
ADAM Metallopeptidase Domain 9
- ADAMTS :
-
A Disintegrin And Metalloproteinase With Thrombospondin Motifs
- AGAP2 :
-
ArfGAP With GTPase Domain, Ankyrin Repeat And PH Domain 2
- AGEs :
-
Advanced Glycation end Products
- AGO2 :
-
Argonaute-2
- Akt :
-
Protein Kinase B
- ALI :
-
Acute Lung Injury
- alpha-SMA :
-
α-Smooth Muscle Actin
- AMH :
-
Anti Müllerain Hormon
- AMI :
-
Acute Myocardial Infarction
- AML12 :
-
Alpha Mouse Liver 12
- ASK1 :
-
Signal-Regulating Kinase 1
- Bad :
-
Bcl-2-Antagonist Of Cell Death Protein
- BALF :
-
Bronchoalveolar Lavage Fluid
- Bax :
-
Bcl-2 Associated X Protein
- BBB :
-
Basso Beattie Bresnahan
- Bcl-2 :
-
B-Cell CLL/Lymphoma 2
- BIP :
-
Binding-Immunoglobulin Protein
- BMD :
-
Bone Mineral Density
- BMECs :
-
Brain Microvascular Endothelial Cells
- BMP-2 :
-
Bone Morphogenetic Protein 2
- BMS :
-
Basso Mouse Scale
- BMSCs :
-
Bone Marrow Mesenchymal Stem Cells
- CaMKII :
-
Calcium/Calmodulin-Dependent Protein Kinase II
- CCNE1 :
-
Cyclin E1
- CD :
-
Clusters of Differentiations
- CDK-4 :
-
Cyclin Dependent Kinase 4
- CFA :
-
Complete Freund’s Adjuvant
- COL10A1 :
-
Collagen type X alpha 1 chain
- COL2A1 :
-
Collagen Type II Alpha 1 Chain
- COL4A1 :
-
Collagen Type IV Alpha 1 Chain
- COL9A1 :
-
Collagen Type IX Alpha 1 Chain
- COMP :
-
Cartilage Oligomeric Matrix Protein
- COX2 :
-
Cyclooxygenase-2
- CREB :
-
CAMP Responsive Element Binding Protein
- CTGF :
-
Connective Tissue Growth Factor
- CTX :
-
Cyclophosphamide
- CYP2J2 :
-
Cytochrome P450 2J2
- DCX :
-
Doublecortin
- DDIT4 :
-
DNA Damage Inducible Transcript 4
- DFO :
-
Deferoxamine
- DGCR8 :
-
DiGeorge Syndrome Critical Region gene 8
- DMBA :
-
7,12-Dimetilbenz [a] antrasen
- DMOG :
-
Dimethyl Oxaloylglycine
- E2 :
-
Estradiol
- EBV :
-
Epstein-Barr Virus
- ECM :
-
Extracellular Matrix
- EGFR :
-
Epidermal Growth Factor Receptor
- EGM-2 :
-
Endothelial Cell Growth Medium
- EMT :
-
Epithelial Mesenchymal Transition
- ERK :
-
Extracellular Signal-Regulated Kinase
- ESCRT :
-
Endosomal Sorting Complex Required for Transport”
- ESM1 :
-
Endothelial Cell-Specific Molecule-1
- EZH2 :
-
Enhancer of Zeste Homolog 2
- FasL :
-
Fas Ligand
- FFT :
-
Foot False Test
- FLS :
-
Fibroblast-Like Synoviocytes
- FOXA2 :
-
Forkhead Box protein A2
- FSH :
-
Follicle Stimulating Hormone
- FSP1 :
-
Ferroptosis Suppressor Protein 1
- GAP43 :
-
Growth Associated Protein 43
- GFAP :
-
Glial Fibrillary Acidic Protein
- Gli3 :
-
GLI Family Zinc Finger 3
- GSK-3 beta :
-
Glycogen Synthase Kinase 3 beta
- GVHD :
-
Graft Versus Host Disease
- H/R :
-
Hypoxia/Reoxygenation
- HDAC3 :
-
Histone Deacetylase 3
- HGF :
-
Hepatocyte Growth Factor
- HHS :
-
Histological Hepatitis Score
- HIF1-α :
-
Hypoxia-Inducible Factor 1-Alpha
- HSP :
-
Heat Shock Protein
- HUVECs :
-
Human Umbilical Vein Endothelial Cells
- I/R :
-
Ischemia/Reperfusion
- ICHÇ :
-
Intracerebral Hemorrhage
- IkB-alpha :
-
IkappaBalpha
- INPP4B :
-
Inositol Polyphosphate-4-Phosphatase Type II B
- IRF2 :
-
Interferon Regulatory Factor 2
- ITGA2 :
-
Integrin Subunit Alpha 2b
- IVD :
-
Intravertebral Disc
- iNOS :
-
Inducible Nitric Oxide Synthase
- JNK :
-
JUN N-Terminal Kinase
- KDR :
-
Kinase Insert Domain Receptor
- KGF :
-
Keratinocyte Growth Factor
- KIM1 :
-
Kidney Injury Molecule 1
- LAD :
-
Left Anterior Descending Coronary Artery Ligation
- LCN2 :
-
Lipocalin2
- LDH :
-
Lactate Dehydrogenase
- LH :
-
luteinizing Hormone
- LMP1 :
-
Latent Membrane Protein 1
- LPS :
-
Lipopolysaccharide
- LVEDD :
-
Left Ventricular End Diastolic Dimension
- LVEDP :
-
Left Ventricular End Diastolic Pressure
- LVEF :
-
Left Ventricular Ejection Fraction
- LVESD :
-
Left Ventricular End Systolic Dimension
- LVFS :
-
Left Ventricular Fractional Shortening
- LVSP :
-
Left Ventricular Systolic Pressure
- MAPK :
-
Mitogen-Activated Protein Kinase
- MCAO :
-
Middle Cerebral Artery Occlusion
- MCP-1 :
-
Monocyte Chemotactic Protein 1
- MDA :
-
Malondialdehyde
- MDI :
-
Motor Deficit Index
- MI :
-
Myocardial Infarction
- MLS :
-
Macrophage-Like Synoviocytes
- MMP14 :
-
Matrix Metallopeptidase 14
- mNSS :
-
Modified Neurologic Severity Score
- MPO :
-
Myeloperoxidase
- MSCs :
-
Mesenchymal Stem Cells
- mTOR :
-
Mammalian Target of Rapamycin
- MVBs :
-
Multi-Vesicular Bodies
- ncRNA :
-
noncoding RNAs
- NeuN :
-
Neuronal Nuclei
- NF :
-
Neurofibromin
- NF-200 :
-
Neurofilament 200
- NFH :
-
Neurofilament Heavy
- NF-kB :
-
Nuclear Factor-kappa B
- NLRP3 :
-
NLR Family Pyrin Domain Containing 3
- NOX4 :
-
NADPH Oksidaz 4
- NPC :
-
Neuroprogenic Cells
- NSCLC :
-
Non-Small Cell Lung Cancer
- OA :
-
Osteoarthiritis
- OGD :
-
Oxygene Glucose Deprivation
- OPMD :
-
Oral Potentially Malignant Disorders
- P38MAPK :
-
P38 MAP Kinase
- PACT :
-
Protein Activator of Interferon-Induced Protein Kinase
- PARP :
-
Poly (ADP-ribose) Polymerase
- PCNA :
-
Proliferating Cell Nuclear Antigen
- PDCD4 :
-
Programmed Cell Death 4
- Peli1 :
-
Pellino-1
- PGE2 :
-
Prostaglandin E2
- PI3K :
-
Phosphoinositide 3-Kinase
- POF :
-
Premature Ovarian Failure
- pre-miRNA :
-
Precursor miRNA
- pri-miRNAs :
-
Primary miRNAs
- PTEN :
-
Phosphatase And Tensin Homolog
- PTGS2 :
-
Prostaglandin-Endoperoxide Synthase 2
- RA :
-
Rheumatoid Arthritis
- RABEPK :
-
Rab Effector Protein with Kelch Motifs
- RAC2 :
-
Rac Family Small GTPase 2
- RASA1 :
-
RAS P21 Protein Activator 1
- RhoA :
-
Ras Homolog Family Member A
- RHPN2 :
-
Rhophilin Rho GTPase Binding Protein 2
- RISC :
-
RNA Induced Silencing Complex
- ROS :
-
Reactive Oxygen Species
- RUNX2 :
-
RUNX Family Transcription Factor 2
- RVG+Lamp2b) :
-
Rabies Virus Glycoprotein + Lysosome-Associated Membrane Glycoprotein 2b
- SAA3 :
-
Serum Amyloid A3
- SAH :
-
Subarachnoid Hemorrhage
- SCI :
-
Spinal Cord Injury
- SCID :
-
Severe Combined Immunodeficiency
- SEMA3A :
-
Semaphorin 3A
- SIRT7 :
-
Sirtun 7
- SMSCs :
-
Synovial Mesenchymal Stem Cells
- SNAIL1 :
-
Snail Family Transcriptional Repressor 1
- SOD :
-
Superoxide Dismutase
- SOX2 :
-
SRY-Box Transcription Factor 2
- SOX9 :
-
SRY-Box Transcription Factor 9
- SP1 :
-
Specificity Protein 1
- STAT3 :
-
Signal Transducer And Activator Of Transcription 3
- TFF3 :
-
Trefoil Factor-3
- TGF-beta 1 :
-
Transforming Growth Factor Beta 1
- TGF-beta :
-
Transforming Growth Factor Beta
- TGF-BR1 :
-
Transforming Growth Factor Beta (TGF-beta) Receptor Type 1
- TNBS :
-
2,4,6-Trinitrobenzene Sulfonic Acid
- TNF-alpha :
-
Tumor Necrosis Factor Alpha
- TRBP :
-
Transactivation Response Element RNA-Binding Protein
- Trx :
-
Thioreductin
- TXNIP :
-
Thioreduxin- Interacting Protein
- UUO :
-
Unilateral Ureteral Obstruction
- VEGFA :
-
Vascular Endothelial Growth Factor A
- VEGFR :
-
Vascular Endothelial Growth Factor Receptor
- VPA :
-
Valproic Acid
- VSMC :
-
Vascular Smooth Muscle Cells
- WNT5A :
-
Wnt Family Member 5A
- XPO5 :
-
Exportin-5
- ZEB :
-
Zinc Finger E-Box Binding Homeobox
References
Zhao, P., Xiao, L., Peng, J., Qian, Y. Q., & Huang, C. C. (2018). Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. European Review for Medical and Pharmacological Sciences, 22(12), 3962–3970.
Ratajczak, M. Z., Zuba-Surma, E. K., Wojakowski, W., Ratajczak, J., & Kucia, M. (2008). Bone marrow - home of versatile stem cells. Transfusion Medicine and Hemotherapy, 35(3), 248–259.
Haider, K. H., & Aramini, B. (2020). Mircrining the injured heart with stem cell-derived exosomes: An emerging strategy of cell-free therapy. Stem Cell Research & Therapy, 11(1), 23.
Liu, H., Liang, Z., Wang, F., Zhou, C., Zheng, X., Hu, T., et al. (2019). Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight, 4(24) Pii: 131273.
Zuo, R., Liu, M., Wang, Y., Li, J., Wang, W., Wu, J., Sun, C., Li, B., Wang, Z., Lan, W., Zhang, C., Shi, C., & Zhou, Y. (2019). BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Research & Therapy, 10(1), 30.
Squillaro, T., Peluso, G., & Galderisi, U. (2016). Clinical trials with Mesenchymal stem cells: An update. Cell Transplantation, 25(5), 829–848.
Brown, C., McKee, C., Bakshi, S., Walker, K., Hakman, E., Halassy, S., Svinarich, D., Dodds, R., Govind, C. K., & Chaudhry, G. R. (2019). Mesenchymal stem cells: Cell therapy and regeneration potential. Journal of Tissue Engineering and Regenerative Medicine, 13(9), 1738–1755.
Wang, S., Zhu, R., Li, H., Li, J., Han, Q., & Zhao, R. C. (2019). Mesenchymal stem cells and immune disorders: From basic science to clinical transition. Frontiers in Medicine, 13(2), 138–151.
Reinders, M. E. J., van Kooten, C., Rabelink, T. J., & de Fijter, J. W. (2018). Mesenchymal stromal cell therapy for solid organ transplantation. Transplantation, 102(1), 35–43.
Wilson, A., Webster, A., & Genever, P. (2019). Nomenclature and heterogeneity: Consequences for the use of mesenchymal stem cells in regenerative medicine. Regenerative Medicine, 14(6), 595–611.
Li, C., Jiao, G., Wu, W., Wang, H., Ren, S., Zhang, L., Zhou, H., Liu, H., & Chen, Y. (2019). Exosomes from bone marrow Mesenchymal stem cells inhibit neuronal apoptosis and promote motor function recovery via the Wnt/β-catenin signaling pathway. Cell Transplantation, 28(11), 1373–1383.
Mendt, M., Rezvani, K., & Shpall, E. (2019). Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplantation, 54(Suppl 2), 789–792.
Yu, H., Cheng, J., Shi, W., Ren, B., Zhao, F., Shi, Y., Yang, P., Duan, X., Zhang, J., Fu, X., Hu, X., & Ao, Y. (2020). Bone marrow mesenchymal stem cell derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomaterialia, 106, 328–341.
Huang, P., Wang, L., Li, Q., Xu, J., Xu, J., Xiong, Y., Chen, G., Qian, H., Jin, C., Yu, Y., Liu, J., Qian, L., & Yang, Y. (2019). Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Research & Therapy, 10(1), 300.
Bian, S., Zhang, L., Duan, L., Wang, X., Min, Y., & Yu, H. (2014). Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of Molecular Medicine (Berlin, Germany), 92(4), 387–397.
Ding, J., Wang, X., Chen, B., Zhang, J., & Xu, J. (2019). Exosomes derived from human bone marrow Mesenchymal stem cells stimulated by Deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. BioMed Research International, 2019, 9742765.
Liang, B., Liang, J. M., Ding, J. N., Xu, J., Xu, J. G., & Chai, Y. M. (2019). Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell derived exosomes enhance boneregeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Research & Therapy, 10(1), 335.
Alzahrani, F. A. (2019). Melatonin improves therapeutic potential of mesenchymal stem cells derived exosomes against renal ischemia-reperfusion injury in rats. American Journal of Translational Research, 11(5), 2887–28907.
Alzahrani, F., Saadeldin, I., & Alkarim, S. (2018). Ameliorative effect of mesenchymal stem cells-derived exosomes on diethylnitrosamine-induced liver injury in albino rats. International Journal of Pharmacology, 14, 1128–1135.
Liu, J., Chen, T., Lei, P., Tang, X., & Huang, P. (2019). Exosomes released by bone marrow Mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. International Journal of Medical Sciences, 16(9), 1238–1244.
Fang, S., Li, Y., & Chen, P. (2018). Osteogenic effect of bone marrow mesenchymal stem cell-derived exosomes on steroid-induced osteonecrosis of the femoral head. Drug Design, Development and Therapy, 13, 45–55.
Miao, C., Lei, M., Hu, W., Han, S., & Wang, Q. (2017). A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Research & Therapy, 8, 242.
Johnstone, R. M., Adam, M., Hammond, J., Orr, L., & Turbide, C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of Biological Chemistry, 262(19), 9412–9420.
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F., Atkin-Smith, G. K., Ayre, D. C., Bach, J. M., Bachurski, D., Baharvand, H., Balaj, L., Baldacchino, S., Bauer, N. N., Baxter, A. A., Bebawy, M., Beckham, C., Bedina Zavec, A., Benmoussa, A., Berardi, A. C., Bergese, P., Bielska, E., Blenkiron, C., Bobis-Wozowicz, S., Boilard, E., Boireau, W., Bongiovanni, A., Borràs, F. E., Bosch, S., Boulanger, C. M., Breakefield, X., Breglio, A. M., Brennan, M. Á., Brigstock, D. R., Brisson, A., Broekman, M. L. D., Bromberg, J. F., Bryl-Górecka, P., Buch, S., Buck, A. H., Burger, D., Busatto, S., Buschmann, D., Bussolati, B., Buzás, E. I., Byrd, J. B., Camussi, G., Carter, D. R. F., Caruso, S., Chamley, L. W., Chang, Y. T., Chen, C., Chen, S., Cheng, L., Chin, A. R., Clayton, A., Clerici, S. P., Cocks, A., Cocucci, E., Coffey, R. J., Cordeiro-da-Silva, A., Couch, Y., Coumans, F. A. W., Coyle, B., Crescitelli, R., Criado, M. F., D’Souza-Schorey, C., Das, S., Datta Chaudhuri, A., de Candia, P., de Santana Jr., E. F., de Wever, O., del Portillo, H. A., Demaret, T., Deville, S., Devitt, A., Dhondt, B., di Vizio, D., Dieterich, L. C., Dolo, V., Dominguez Rubio, A. P., Dominici, M., Dourado, M. R., Driedonks, T. A. P., Duarte, F. V., Duncan, H. M., Eichenberger, R. M., Ekström, K., el Andaloussi, S., Elie-Caille, C., Erdbrügger, U., Falcón-Pérez, J. M., Fatima, F., Fish, J. E., Flores-Bellver, M., Försönits, A., Frelet-Barrand, A., Fricke, F., Fuhrmann, G., Gabrielsson, S., Gámez-Valero, A., Gardiner, C., Gärtner, K., Gaudin, R., Gho, Y. S., Giebel, B., Gilbert, C., Gimona, M., Giusti, I., Goberdhan, D. C. I., Görgens, A., Gorski, S. M., Greening, D. W., Gross, J. C., Gualerzi, A., Gupta, G. N., Gustafson, D., Handberg, A., Haraszti, R. A., Harrison, P., Hegyesi, H., Hendrix, A., Hill, A. F., Hochberg, F. H., Hoffmann, K. F., Holder, B., Holthofer, H., Hosseinkhani, B., Hu, G., Huang, Y., Huber, V., Hunt, S., Ibrahim, A. G. E., Ikezu, T., Inal, J. M., Isin, M., Ivanova, A., Jackson, H. K., Jacobsen, S., Jay, S. M., Jayachandran, M., Jenster, G., Jiang, L., Johnson, S. M., Jones, J. C., Jong, A., Jovanovic-Talisman, T., Jung, S., Kalluri, R., Kano, S. I., Kaur, S., Kawamura, Y., Keller, E. T., Khamari, D., Khomyakova, E., Khvorova, A., Kierulf, P., Kim, K. P., Kislinger, T., Klingeborn, M., Klinke II, D. J., Kornek, M., Kosanović, M. M., Kovács, Á. F., Krämer-Albers, E. M., Krasemann, S., Krause, M., Kurochkin, I. V., Kusuma, G. D., Kuypers, S., Laitinen, S., Langevin, S. M., Languino, L. R., Lannigan, J., Lässer, C., Laurent, L. C., Lavieu, G., Lázaro-Ibáñez, E., le Lay, S., Lee, M. S., Lee, Y. X. F., Lemos, D. S., Lenassi, M., Leszczynska, A., Li, I. T. S., Liao, K., Libregts, S. F., Ligeti, E., Lim, R., Lim, S. K., Linē, A., Linnemannstöns, K., Llorente, A., Lombard, C. A., Lorenowicz, M. J., Lörincz, Á. M., Lötvall, J., Lovett, J., Lowry, M. C., Loyer, X., Lu, Q., Lukomska, B., Lunavat, T. R., Maas, S. L. N., Malhi, H., Marcilla, A., Mariani, J., Mariscal, J., Martens-Uzunova, E. S., Martin-Jaular, L., Martinez, M. C., Martins, V. R., Mathieu, M., Mathivanan, S., Maugeri, M., McGinnis, L. K., McVey, M. J., Meckes Jr., D. G., Meehan, K. L., Mertens, I., Minciacchi, V. R., Möller, A., Møller Jørgensen, M., Morales-Kastresana, A., Morhayim, J., Mullier, F., Muraca, M., Musante, L., Mussack, V., Muth, D. C., Myburgh, K. H., Najrana, T., Nawaz, M., Nazarenko, I., Nejsum, P., Neri, C., Neri, T., Nieuwland, R., Nimrichter, L., Nolan, J. P., Nolte-’t Hoen, E. N. M., Noren Hooten, N., O’Driscoll, L., O’Grady, T., O’Loghlen, A., Ochiya, T., Olivier, M., Ortiz, A., Ortiz, L. A., Osteikoetxea, X., Østergaard, O., Ostrowski, M., Park, J., Pegtel, D. M., Peinado, H., Perut, F., Pfaffl, M. W., Phinney, D. G., Pieters, B. C. H., Pink, R. C., Pisetsky, D. S., Pogge von Strandmann, E., Polakovicova, I., Poon, I. K. H., Powell, B. H., Prada, I., Pulliam, L., Quesenberry, P., Radeghieri, A., Raffai, R. L., Raimondo, S., Rak, J., Ramirez, M. I., Raposo, G., Rayyan, M. S., Regev-Rudzki, N., Ricklefs, F. L., Robbins, P. D., Roberts, D. D., Rodrigues, S. C., Rohde, E., Rome, S., Rouschop, K. M. A., Rughetti, A., Russell, A. E., Saá, P., Sahoo, S., Salas-Huenuleo, E., Sánchez, C., Saugstad, J. A., Saul, M. J., Schiffelers, R. M., Schneider, R., Schøyen, T. H., Scott, A., Shahaj, E., Sharma, S., Shatnyeva, O., Shekari, F., Shelke, G. V., Shetty, A. K., Shiba, K., Siljander, P. R. M., Silva, A. M., Skowronek, A., Snyder II, O. L., Soares, R. P., Sódar, B. W., Soekmadji, C., Sotillo, J., Stahl, P. D., Stoorvogel, W., Stott, S. L., Strasser, E. F., Swift, S., Tahara, H., Tewari, M., Timms, K., Tiwari, S., Tixeira, R., Tkach, M., Toh, W. S., Tomasini, R., Torrecilhas, A. C., Tosar, J. P., Toxavidis, V., Urbanelli, L., Vader, P., van Balkom, B. W. M., van der Grein, S. G., van Deun, J., van Herwijnen, M. J. C., van Keuren-Jensen, K., van Niel, G., van Royen, M. E., van Wijnen, A. J., Vasconcelos, M. H., Vechetti Jr., I. J., Veit, T. D., Vella, L. J., Velot, É., Verweij, F. J., Vestad, B., Viñas, J. L., Visnovitz, T., Vukman, K. V., Wahlgren, J., Watson, D. C., Wauben, M. H. M., Weaver, A., Webber, J. P., Weber, V., Wehman, A. M., Weiss, D. J., Welsh, J. A., Wendt, S., Wheelock, A. M., Wiener, Z., Witte, L., Wolfram, J., Xagorari, A., Xander, P., Xu, J., Yan, X., Yáñez-Mó, M., Yin, H., Yuana, Y., Zappulli, V., Zarubova, J., Žėkas, V., Zhang, J. Y., Zhao, Z., Zheng, L., Zheutlin, A. R., Zickler, A. M., Zimmermann, P., Zivkovic, A. M., Zocco, D., & Zuba-Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles., 7(1), 1535750.
Lu, K., Li, H. Y., Yang, K., Wu, J. L., Cai, X. W., Zhou, Y., & Li, C. Q. (2017). Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In vitro study on exosomes in interaction of nucleus pulposus cells and bone marow mesenchymal stem cells. Stem Cell Research & Therapy, 8(1), 108.
Yokoi, A., Villar-Prados, A., Oliphint, P. A., Zhang, J., Song, X., De Hoff, P., Morey, R., Liu, J., Roszik, J., Clise-Dwyer, K., Burks, J. K., O'Halloran, T. J., Laurent, L. C., & Sood, A. K. (2019). Mechanisms of nuclear content loading to exosomes. Sci Adv, 5(11), eaax8849.
Ouyang, X., Han, X., Chen, Z., Fang, J., Huang, X., & Wei, H. (2018). MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Research & Therapy, 9(1), 246.
Burrello, J., Monticone, S., Gai, C., Gomez, Y., Kholia, S., & Giovanni, C. G. (2016). Stem cell-derived extracellular vesicles and immune-modulation. Frontiers in Cell and Development Biology, 4, 83.
Altanerova, U., Jakubechova, J., Benejova, K., Priscakova, P., Pesta, M., Pitule, P., Topolcan, O., Kausitz, J., Zduriencikova, M., Repiska, V., & Altaner, C. (2019). Prodrug suicide gene therapy for cancer targeted intracellular by mesenchymal stem cell exosomes. International Journal of Cancer, 144(4), 897–908.
Shimaoka, M., Kawamoto, E., Gaowa, A., Okamoto, T., & Park, E. J. (2019). Connexins and Integrins in Exosomes. Cancers (Basel), 11(1), 106.
Tsao, C. R., Liao, M. F., Wang, M. H., Cheng, C. M., & Chen, C. H. (2014). Mesenchymal stem cell derived Exosomes: A new Hope for the treatment of cardiovascular disease? Zhonghua Minguo Xin Zang Xue Hui Za Zhi, 30(5), 395–400.
Forsberg, M. H., Kink, J. A., Hematti, P., & Capitinin, C. M. (2020). Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front Cell Dev Biol.
Ma, Z. J., Yang, J. J., Lu, Y. B., Liu, Z. Y., & Wang, X. X. (2020). Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells, 12(8), 814–840.
Zhuang, X., Xiang, X., Grizzle, W., Sun, D., Zhang, S., Axtell, R. C., Ju, S., Mu, J., Zhang, L., Steinman, L., Miller, D., & Zhang, H. G. (2011). Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Molecular Therapy, 19, 1769–1779.
Xie, X., Wu, H., Li, M., Chen, X., Xu, X., Ni, W., Lu, C., Ni, R., Bao, B., & Xiao, M. (2019). Progress in the application of exosomes as therapeutic vectors in tumor-targeted therapy. Cytotherapy, 21(5), 509–524.
Ha, D., Yang, N., & Nadithe, V. (2016). Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharmaceutica Sinica B, 6(4), 287–296.
Baharlooi, H., Azimi, M., Salehi, Z., & Izad, M. (2020). Mesenchymal stem cell-derived Exosomes: A promising therapeutic ace card to address autoimmune diseases. Int J Stem Cells, 13(1), 13–23.
Rezaie, J., Ajezi, S., Avci, Ç. B., Karimipour, M., Geranmayeh, M. H., Nourazarian, A., Sokullu, E., Rezabakhsh, A., & Rahbarghazi, R. (2018). Exosomes and their application in biomedical field: Difficulties and advantages. Molecular Neurobiology, 55(4), 3372–3393.
Zhang, L., Jiao, G., Ren, S., Zhang, X., Li, C., Wu, W., Wang, H., Liu, H., Zhou, H., & Chen, Y. (2020). Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Research & Therapy, 11(1), 38.
Wei, H., Chen, J., Wang, S., Fu, F., Zhu, X., Wu, C., Liu, Z., Zhong, G., & Lin, J. (2019). A Nanodrug consisting of doxorubicin and exosome derived from Mesenchymal stem cells for osteosarcoma treatment In vitro. International Journal of Nanomedicine, 14, 8603–8610.
Li, Z., Liu, F., He, X., Yang, X., Shan, F., & Feng, J. (2019). Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. International Immunopharmacology, 67, 268–280.
Yeo, R. W., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., et al. (2013). Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Advanced Drug Delivery Reviews, 65(3), 336–341.
Eldh, M., Ekstrom, K., Valadi, H., Sjostrand, M., Olsson, B., Jernas, M., et al. (2010). Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One, 5(12), e15353.
Meckes Jr., D. G., Shair, K. H., Marquitz, A. R., Kung, C. P., Edwards, R. H., & Raab-Traub, N. (2010). Human tumor virus utilizes exosomes for intercellular communication. Proceedings of the National Academy of Sciences of the United States of America, 107(47), 20370–20375.
Xie, L., Wang, J., Zhang, Y., Chen, H., Lin, D., Ding, J., Xuan, J., Chen, Q., & Cai, L. (2019). The effects of local injection of exosomes derived from BMSCs on random skin flap in rats. American Journal of Translational Research, 11(11), 7063–7073.
Hou, K., Li, G., Zhao, J., Xu, B., Zhang, Y., Yu, J., & Xu, K. (2020). Bone mesenchymal stem cell-derived exosomal microRNA-29b-3p prevents hypoxic-ischemic injury in rat brain by activating the PTEN-mediated Akt signaling pathway. Journal of Neuroinflammation, 17(1), 46.
Lu, Y., Zhou, Y., Zhang, R., Wen, L., Wu, K., Li, Y., Yao, Y., Duan, R., & Jia, Y. (2019). Bone Mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Frontiers in Neuroscience, 13, 209.
Zeng, Q., Zhou, Y., Liang, D., He, H., Liu, X., Zhu, R., Zhang, M., Luo, X., Wang, Y., & Huang, G. (2020). Exosomes secreted from bone marrow Mesenchymal stem cells attenuate oxygen-glucose deprivation/Reoxygenation-induced Pyroptosis in PC12 cells by promoting AMPK-dependent Autophagic flux. Frontiers in Cellular Neuroscience, 14, 182.
Reis, L. A., Borges, F. T., Simões, M. J., Borges, A. A., Sinigaglia-Coimbra, R., & Schor, N. (2012). Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One, 7(9), e44092.
Zhao, S., Liu, Y., & Pu, Z. (2019). Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Design, Development and Therapy, 13, 2887–2897.
Yu, M., Liu, W., Li, J., Lu, J., Lu, H., Jia, W., & Liu, F. (2020). Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Research & Therapy, 11(1), 350.
Bruno, S., Tapparo, M., Collino, F., Chiabotto, G., Deregibus, M. C., Soares Lindoso, R., Neri, F., Kholia, S., Giunti, S., Wen, S., Quesenberry, P., & Camussi, G. (2017). Renal regenerative potential of different extracellular vesicle populations derived from bone marrow Mesenchymal stromal cells. Tissue Engineering. Part A, 23(21–22), 1262–1273.
El Andaloussi, S., Lakhal, S., Mager, I., & Wood, M. J. (2013). Exosomes for targeted siRNA delivery across biological barriers. Advanced Drug Delivery Reviews, 65(3), 391–397.
Pedroza-Torres, A., Romero-Córdoba, S. L., Justo-Garrido, M., Salido-Guadarrama, I., Rodríguez-Bautista, R., Montaño, S., Muñiz-Mendoza, R., Arriaga-Canon, C., Fragoso-Ontiveros, V., Álvarez-Gómez, R. M., Hernández, G., & Herrera, L. A. (2019). MicroRNAs in tumor cell metabolism: Roles and therapeutic opportunities. Frontiers in Oncology, 9, 1404.
Syed, S. N., & Brüne, B. (2020). MicroRNAs as emerging regulators of signaling in the tumor microenvironment. Cancers (Basel), 12(4).
Ding, L., Gu, H., Xiong, X., Ao, H., Cao, J., Lin, W., et al. (2019). MicroRNAs involved in carcinogenesis, prognosis, therapeutic resistance and applications in human triple-negative breast Cancer. Cells, 8(12) pii: E1492.
Sohel, M. M. H. (2020). Circulating microRNAs as biomarkers in cancer diagnosis. Life Sciences, 248, 117473.
Behl, T., Kumar, C., Makkar, R., Gupta, A., & Sachdeva, M. (2020). Intercalating the role of MicroRNAs in Cancer: As enemy or protector. Asian Pacific Journal of Cancer Prevention, 21(3), 593–298.
Tüfekci, K. U., Meuwissen, R. L., & Genç, S. (2014). The role of microRNAs in biological processes. Methods in Molecular Biology, 1107, 15–31.
Chen, L., Heikkinen, L., Wang, C., Yang, Y., Sun, H., & Wong, G. (2019). Trends in the development of miRNA bioinformatics tools. Briefings in Bioinformatics, 20(5), 1836–1852.
Liu, W., & Wang, X. (2019). Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biology, 20(1), 18.
Van Vu, T., & Do, V. N. (2017). Customization of artificial MicroRNA design. In S. Rani (Ed.), MicroRNA profiling. Methods in molecular biology (Vol. 1509). New York, NY: Humana Press.
Fan, J., Feng, Y., Zhang, R., Zhang, W., Shu, Y., Zeng, Z., Huang, S., Zhang, L., Huang, B., Wu, D., Zhang, B., Wang, X., Lei, Y., Ye, Z., Zhao, L., Cao, D., Yang, L., Chen, X., Liu, B., Wagstaff, W., He, F., Wu, X., Zhang, J., Wolf, J. M., Lee, M. J., Haydon, R. C., Luu, H. H., Huang, A., He, T. C., & Yan, S. (2020). A simplified system for the effective expression and delivery of functional mature microRNAs in mammalian cells. Cancer Gene Therapy, 27(6), 424–437.
Zhang, N., Zhang, D., Chen, S. L., Gong, B. Q., Guo, Y., Xu, L., Zhang, X. N., & Li, J. F. (2018). Engineering artificial MicroRNAs for multiplex gene silencing and simplified transgenic screen. Plant Physiology, 178(3), 989–1001.
Calloni, R., & Bonatto, D. (2015). Scaffolds for artificial miRNA expression in animal cells. Hum Gene Ther Methods, 26(5), 162–174.
Borel, F., Kay, M. A., & Mueller, C. (2014). Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Molecular Therapy, 22(4), 692–701.
Herrera-Carrillo, E., Liu, Y. P., & Berkhout, B. (2017). Improving miRNA delivery by optimizing miRNA expression cassettes in diverse virus vectors. Hum Gene Ther Methods, 28(4), 177–190.
Liu, Y. P., & Berkhout, B. (2011). miRNA cassettes in viral vectors: Problems and solutions. Biochimica et Biophysica Acta, 1809(11–12), 732–745.
Yang, N. (2015). An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig, 5(4), 179–181.
Vannucci, L., Lai, M., Chiuppesi, F., Ceccherini-Nelli, L., & Pistello, M. (2013). Viral vectors: A look back and ahead on gene transfer technology. The New Microbiologica, 36(1), 1–22.
Rousset, F., Salmon, P., Bredl, S., Cherpin, O., Coelho, M., Myburgh, R., Alessandrini, M., Perny, M., Roccio, M., Speck, R. F., Senn, P., & Krause, K. H. (2019). Optimizing synthetic miRNA Minigene architecture for efficient miRNA hairpin concatenation and multi-target gene knockdown. Mol Ther Nucleic Acids, 14, 351–363.
Fu, Y., Chen, J., & Huang, Z. (2019). Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA, 1, 24.
Paul, S., Vázquez, L. A. B., Uribe, S. P., Reyes-Pérez, P. R., & Sharma, A. (2020). Current status of microRNA-based therapeutic approaches in neurodegenerative disorders. Cell, 9(7), 1698.
Xu, F., Xiang, Q., Huang, J., Chen, Q., Yu, N., Long, X., & Zhou, Z. (2019). Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Research & Therapy, 10(1), 106.
Hyun, J., Wang, S., Kim, J., Kim, G. J., & Jung, Y. (2015). MicroRNA125b-mediated hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Scientific Reports, 5, 14135.
Wang, K., Jiang, Z., Webster, K. A., Chen, J., Hu, H., Zhou, Y., Zhao, J., Wang, L., Wang, Y., Zhong, Z., Ni, C., Li, Q., Xiang, C., Zhang, L., Wu, R., Zhu, W., Yu, H., Hu, X., & Wang, J. (2017). Enhanced Cardioprotection by human endometrium Mesenchymal stem cells driven by Exosomal MicroRNA-21. Stem Cells Translational Medicine, 6(1), 209–222.
Ding, Y., Cao, F., Sun, H., Wang, Y., Liu, S., Wu, Y., Cui, Q., Mei, W. T., & Li, F. (2019). Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Letters, 442, 351–361.
Sharif, S., Ghahremani, M. H., & Soleimani, M. (2018). Delivery of exogenous miR-124 to Glioblastoma multiform cells by Wharton's jelly Mesenchymal stem cells decreases cell proliferation and migration, and confers Chemosensitivity. Stem Cell Reviews and Reports, 14(2), 236–246.
Zhang, L., Wang, J., Fu, Z., Ai, Y., Li, Y., Wang, Y., & Wang, Y. (2019). Sevoflurane suppresses migration and invasion of glioma cells by regulating miR-146b-5p and MMP16. Artif Cells Nanomed Biotechnol, 47(1), 3306–3314.
Katakowski, M., Buller, B., Zheng Xi, L. Y., Rogers, T., Osobamiro, O., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335(1), 201–204.
Lu, J., Ji, H., Tang, H., & Xu, Z. (2018). microRNA-124a suppresses PHF19 over-expression, EZH2 hyper-activation, and aberrant cell proliferation in human glioma. Biochemical and Biophysical Research Communications, 503(3), 1610–1617.
Lang, F. M., Hossain, A., Gumin, J., Momin, E. N., Shimizu, Y., Ledbetter, D., Shahar, T., Yamashita, S., Parker Kerrigan, B., Fueyo, J., Sawaya, R., & Lang, F. F. (2018). Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-Oncology, 20(3), 380–390.
Kim, R., Lee, S., Lee, J., Kim, M., KimWJ, L. H. W., et al. (2018). Exosomes derived from microRNA-584 transfected Mesenchymal stem cells: Novel alternative therapeutic vehicles for Cancer therapy. BMB Reports, 51(8), 406–411.
Liu, H., Sun, Y., Qi, X., Gordon, R. E., O'Brien, J. A., Yuan, H., Zhang, J., Wang, Z., Zhang, M., Song, Y., Yu, C., & Gu, C. (2019). EZH2 phosphorylation promotes self-renewal of Glioma stem-like cells through NF-κB methylation. Frontiers in Oncology, 9, 641.
Zhang, Q., Fan, X., Xu, B., Pang, Q., & Teng, L. (2018). miR-133b acts as a tumor suppressor and negatively regulates EMP2 in glioma. Neoplasma, 65(4), 494–504.
Xu, H., Zhao, G., Zhang, Y., Jiang, H., Wang, W., Zhao, D., Hong, J., Yu, H., & Qi, L. (2019). Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Research & Therapy, 10(1), 381.
Yu, L., Gui, S., Liu, Y., Qiu, X., Zhang, G., Zhang, X., et al. (2019). Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging (Albany NY), 11(15), 5300–5318.
Zhang, F., Lu, Y., Wang, M., Zhu, J., Li, J., Zhang, P., et al. (2020). Exosomes derived from human bone marrow mesenchymal stem cells transfer miR-222-3p to suppress acute myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol Cell, Probes, 101513.
Umezu, T., Imanishi, S., Azuma, K., Kobayashi, C., Yoshizawa, S., Ohyashiki, K., & Ohyashiki, J. H. (2017). Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Advances, 1(13), 812–823.
Bao, Q., Liao, X., Li, R., & Ding, N. (2019). KCNQ1OT1 promotes migration and inhibits apoptosis by modulating miR-185-5p/Rab14 axis in oral squamous cell carcinoma. Development, Growth & Differentiation, 61(9), 466–474.
Wang, L., Yin, P., Wang, J., Wang, Y., Sun, Z., Zhou, Y., & Guan, X. (2019). Delivery of mesenchymal stem cells-derived extracellular vesicles with enriched miR-185 inhibits progression of OPMD. Artif Cells Nanomed Biotechnol, 47(1), 2481–2491.
Xie, C., Du, L. Y., Guo, F., Li, X., & Cheng, B. (2019). Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Molecular and Cellular Biochemistry, 458(1–2), 11–26.
Chen, S. L., Ma, M., Yan, L., Xiong, S. H., Liu, Z., Li, S., Liu, T., Shang, S., Zhang, Y. Y., Zeng, H., Xie, H. L., & Zuo, C. H. (2019). Clinical significance of exosomal miR-1231 in pancreatic cancer. Zhonghua Zhong Liu Za Zhi, 41(1), 46–49.
Shang, S., Wang, J., Chen, S., Tian, R., Zeng, H., Wang, L., Xia, M., Zhu, H., & Zuo, C. (2019). Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Medicine, 8(18), 7728–7740.
Wu, D. M., Wen, X., Han, X. R., Wang, S., Wang, Y. J., Shen, M., Fan, S. H., Zhang, Z. F., Shan, Q., Li, M. Q., Hu, B., Lu, J., Chen, G. Q., & Zheng, Y. L. (2019). Bone marrow Mesenchymal stem cell-derived Exosomal MicroRNA-126-3p inhibits pancreatic Cancer development by targeting ADAM9. Mol Ther Nucleic Acids, 16, 229–245.
Xu, Y., Shen, L., Li, F., Yang, J., Wan, X., & Ouyang, M. (2019). microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. Journal of Cellular Physiology, 234(11), 21380–21394.
Roudnicky, F., Poyet, C., Wild, P., Krampitz, S., Negrini, F., Huggenberger, R., Rogler, A., Stohr, R., Hartmann, A., Provenzano, M., Otto, V. I., & Detmar, M. (2013). Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Research, 73(3), 1097–1106.
Cai, H., Yang, X., Gao, Y., Xu, Z., Yu, B., Xu, T., Li, X., Xu, W., Wang, X., & Hua, L. (2019). Exosomal MicroRNA-9-3p secreted from BMSCs Downregulates ESM1 to suppress the development of bladder Cancer. Mol Ther Nucleic Acids, 18, 787–800.
Li, L., & Li, S. (2018). miR-205-5p inhibits cell migration and invasion in prostatic carcinoma by targeting ZEB1. Oncology Letters, 16(2), 1715–1721.
Jiang, S., Mo, C., Guo, S., Zhuang, J., Huang, B., & Mao, X. (2019). Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. Journal of Experimental & Clinical Cancer Research, 38(1), 495.
Che, Y., Shi, X., Shi, Y., Jiang, X., Ai, Q., Shi, Y., Gong, F., & Jiang, W. (2019). Exosomes derived from miR-143-overexpressing MSCs inhibit cell migration and invasion in human prostate Cancer by Downregulating TFF3. Mol Ther Nucleic Acids., 18, 232–244.
Liang, Y., Zhang, D., Li, L., Xin, T., Zhao, Y., Ma, R., & du, J. (2020). Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Research & Therapy, 11(1), 87.
Shimbo, K., Miyaki, S., Ishitobi, H., Kato, Y., Kubo, T., Shimose, S., & Ochi, M. (2014). Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochemical and Biophysical Research Communications, 445(2), 381–387.
Liu, L., Xu, L., Zhang, S., Wang, D., Dong, G., Chen, H., Li, X., Shu, C., & Wang, R. (2018). STF-083010, an inhibitor of XBP1 splicing, attenuates acute renal failure in rats by suppressing endoplasmic reticulum stress-induced apoptosis and inflammation. Experimental Animals, 67(3), 373–382.
Wang, R., Lin, M., Li, L., Li, L., Qi, G., Rong, R., Xu, M., & Zhu, T. (2014). Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi, 94, 3298–3303.
Wang, C., Zhu, G., He, W., Yin, H., Lin, F., Gou, X., & Li, X. (2019). BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. The FASEB Journal, 33(4), 5440–5456.
Tapparo, M., Bruno, S., Collino, F., Togliatto, G., Deregibus, M. C., Provero, P., Wen, S., Quesenberry, P. J., & Camussi, G. (2019). Renal regenerative potential of extracellular vesicles derived from miRNA-engineered Mesenchymal stromal cells. International Journal of Molecular Sciences, 20(10), 2381.
Zhu, G., Pei, L., Lin, F., Yin, H., Li, X., He, W., Liu, N., & Gou, X. (2019). Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. Journal of Cellular Physiology, 234(12), 23736–23749.
Lu, F. B., Chen, D. Z., Chen, L., Hu, E. D., Wu, J. L., Li, H., Gong, Y. W., Lin, Z., Wang, X. D., Li, J., Jin, X. Y., Xu, L. M., & Chen, Y. P. (2019). Attenuation of experimental autoimmune hepatitis in mice with bone Mesenchymal stem cell-derived Exosomes carrying MicroRNA-223-3p. Molecules and Cells, 42(12), 906–918.
Chen, L., Lu, F. B., Chen, D. Z., Wu, J. L., Hu, E. D., Xu, L. M., Zheng, M. H., Li, H., Huang, Y., Jin, X. Y., Gong, Y. W., Lin, Z., Wang, X. D., & Chen, Y. P. (2018). BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Molecular Immunology, 93, 38–46.
Wang, B., Yao, K., Huuskes, B. M., Shen, H. H., Zhuang, J., Godson, C., Brennan, E. P., Wilkinson-Berka, J. L., Wise, A. F., & Ricardo, S. D. (2016). Mesenchymal stem cells deliver exogenous MicroRNA-let7c via Exosomes to attenuate renal fibrosis. Molecular Therapy, 24(7), 1290–1301.
Chen, Y., Ge, W., Xu, L., Qu, C., Zhu, M., Zhang, W., et al. (2012). miR-200b is involved in intestinal fibrosis of Crohn's disease. International Journal of Molecular Medicine, 29(4), 601–606.
Yang, J., Zhou, C. Z., Zhu, R., Fan, H., Liu, X. X., Duan, X. Y., Tang, Q., Shou, Z. X., & Zuo, D. M. (2017). miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. Journal of Gastroenterology and Hepatology, 32(12), 1966–1974.
Yi, X., Wei, X., Lv, H., An, Y., Li, L., Lu, P., Yang, Y., Zhang, Q., Yi, H., & Chen, G. (2019). Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Experimental Cell Research, 383(2), 111454.
Hanna, A., & Frangogiannis, N. G. (2019). The role of the TGF-β superfamily in myocardial infarction. Front Cardiovasc Med, 6, 140.
Shi, B., Wang, Y., Zhao, R., Long, X., Deng, W., & Wang, Z. (2018). Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One, 13(2), e0191616.
Ma, T., Chen, Y., Chen, Y., Meng, Q., Sun, J., Shao, L., et al. (2018). MicroRNA-132 delivered by Mesenchymal stem cell-derived Exosomes, promote angiogenesis in myocardial infarction. Stem Cells International, 2018, 3290372.
Luther, K. M., Haar, L., McGuinness, M., Wang, Y., Lynch Iv, T. L., Phan, A., et al. (2018). Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. Journal of Molecular and Cellular Cardiology, 119, 125–137.
Chen, Q., Liu, Y., Ding, X., Li, Q., Qiu, F., Wang, M., Shen, Z., Zheng, H., & Fu, G. (2020). Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Molecular and Cellular Biochemistry, 465(1–2), 103–114.
Wang, Y., Zhao, R., Liu, D., Deng, W., Xu, G., Liu, W., et al. (2018). Exosomes derived from miR-214-enriched bone marrow-derived Mesenchymal stem cells regulate oxidative damage in cardiac stem cells by targeting CaMKII. Oxidative Medicine and Cellular Longevity, 2018, 4971261.
Li, Y., Yang, R., Guo, B., Zhang, H., Zhang, H., Liu, S., & Li, Y. (2019). Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochemical and Biophysical Research Communications, 514(1), 323–328.
Xu, C., Hu, Y., Hou, L., Ju, J., Li, X., Du, N., et al. (2014). β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. Journal of Molecular and Cellular Cardiology, 75, 111–121.
Chen, Y., Zhao, Y., Chen, W., Xie, L., Zhao, Z. A., Yang, J., Chen, Y., Lei, W., & Shen, Z. (2017). MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Research & Therapy, 8(1), 268.
Barreto, G., Manninen, M. K., & Eklund, K. (2020). Osteoarthritis and toll-like receptors: When innate immunity meets chondrocyte apoptosis. Biology (Basel), 9(4) pii: E65.
Mao, G., Zhang, Z., Huang, Z., Chen, W., Huang, G., Meng, F., Zhang, Z., & Kang, Y. (2017). MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthritis and Cartilage, 25(4), 521–532.
Huang, J., Chen, C., Liang, C., Luo, P., Xia, G., Zhang, L., Wang, X., Wen, Z., Cao, X., & Wu, S. (2020). Dysregulation of the Wnt signaling pathway and synovial stem cell dysfunction in osteoarthritis development. Stem Cells and Development, 29(7), 401–413.
Mao, G., Zhang, Z., Hu, S., Zhang, Z., Chang, Z., Huang, Z., Liao, W., & Kang, Y. (2018). Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Research & Therapy, 9(1), 247.
Liu, F. C., Wang, C. C., Lu, J. W., Lee, C. H., Chen, S. C., Ho, Y. J., et al. (2019). Chondroprotective effects of Genistein against osteoarthritis induced joint inflammation. Nutrients, 11(5) Pii: E1180.
Jin, Z., Ren, J., & Qi, S. (2020). Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. International Immunopharmacology, 78, 105946.
Comertpay, B., & Gov, E. (2020). Identification of key biomolecules in rheumatoid arthritis through the reconstruction of comprehensive disease-specific biological networks. Autoimmunity, 3, 1–11.
Fang, Q., Zhou, C., & Nandakumar, K. S. (2020). Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators of Inflammation, 2020, 3830212.
Liu, Y., Han, Y., Qu, H., Fang, J., Ye, M., & Yin, W. (2019). Correlation of microRNA expression profile with clinical response to tumor necrosis factor inhibitor in treating rheumatoid arthritis patients: A prospective cohort study. Journal of Clinical Laboratory Analysis, 33(7), e22953.
Zheng, J., Zhu, L., Iok In, I., Chen, Y., Jia, N., & Zhu, W. (2020). Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192-5p delays inflammatory response in rheumatoid arthritis. International Immunopharmacology, 78, 105985.
Chen, Z., Wang, H., Xia, Y., Yan, F., & Lu, Y. (2018). Therapeutic potential of Mesenchymal cell-derived miRNA-150-5p-expressing Exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. Journal of Immunology, 201(8), 2472–2482.
Rikhtegar, R., Yousefi, M., Dolati, S., Kasmaei, H. D., Charsouei, S., Nouri, M., & Shakouri, S. K. (2019). Stem cell-based cell therapy for neuroprotection in stroke: A review. Journal of Cellular Biochemistry, 120(6), 8849–8862.
Xiao, Y., Geng, F., Wang, G., Li, X., Zhu, J., & Zhu, W. (2018). Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. Journal of Cellular Biochemistry.
Xin, H., Katakowski, M., Wang, F., Qian, J. Y., Liu, X. S., Ali, M. M., Buller, B., Zhang, Z. G., & Chopp, M. (2017). MicroRNA cluster miR-17-92 cluster in Exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke, 48(3), 747–753.
Hamzei Taj, S., Kho, W., Riou, A., Wiedermann, D., & Hoehn, M. (2016). MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials, 91, 151–165.
Yang, J., Zhang, X., Chen, X., Wang, L., & Yang, G. (2017). Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids, 7, 278–287.
Xin, H., Li, Y., Liu, Z., Wang, X., Shang, X., Cui, Y., Zhang, Z. G., & Chopp, M. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells, 31(12), 2737–2746.
Deng, Y., Chen, D., Gao, F., Lv, H., Zhang, G., Sun, X., Liu, L., Mo, D., Ma, N., Song, L., Huo, X., Yan, T., Zhang, J., & Miao, Z. (2019). Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. Journal of Biological Engineering, 13, 71.
Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., Wang, X., Shang, X., Zhang, Z. G., & Chopp, M. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 30(7), 1556–1564.
Xin, H., Wang, F., Li, Y., Lu, Q. E., Cheung, W. L., Zhang, Y., Zhang, Z. G., & Chopp, M. (2017). Secondary release of Exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with Exosomes harvested from MicroRNA 133b-overexpressing multipotent Mesenchymal stromal cells. Cell Transplantation, 26(2), 243–257.
Lai, N., Wu, D., Liang, T., Pan, P., Yuan, G., Li, X., Li, H., Shen, H., Wang, Z., & Chen, G. (2020). Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. Journal of Neuroinflammation, 17(1), 74.
Shen, H., Yao, X., Li, H., Li, X., Zhang, T., Sun, Q., Ji, C., & Chen, G. (2018). Role of Exosomes derived from miR-133b modified MSCs in an experimental rat model of Intracerebral hemorrhage. Journal of Molecular Neuroscience, 64(3), 421–430.
Theis T, Yoo M, Park CS, Chen J, Kügler S, Gibbs KM, et. al. (2017). Lentiviral delivery of miR-133b improves functional recovery after spinal cord injury in mice. Molecular Neurobiology, 54(6), 4659–4671.
Li, D., Zhang, P., Yao, X., Li, H., Shen, H., Li, X., Wu, J., & Lu, X. (2018). Exosomes derived from miR-133b-modified Mesenchymal stem cells promote recovery after spinal cord injury. Frontiers in Neuroscience, 12, 845.
Yu, T., Zhao, C., Hou, S., Zhou, W., Wang, B., & Chen, Y. (2019). Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Brazilian Journal of Medical and Biological Research, 52(12), e8735.
Cheng, X., Zhang, G., Zhang, L., Hu, Y., Zhang, K., Sun, X., Zhao, C., Li, H., Li, Y. M., & Zhao, J. (2018). Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. Journal of Cellular and Molecular Medicine, 22(1), 261–276.
Ji, W., Jiang, W., Li, M., Li, J., & Li, Z. (2019). miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie., 167, 171–178.
Guo, H., Huang, B., Wang, Y., Zhang, Y., Ma, Q., & Ren, Y. (2020). Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. International Immunopharmacology, 82, 106285.
Zhao, L., Jiang, X., Shi, J., Gao, S., Zhu, Y., Gu, T., & Shi, E. (2019). Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. The Journal of Thoracic and Cardiovascular Surgery, 157(2), 508–517.
Li, C., Li, X., Zhao, B., & Wang, C. (2020). Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch Physiol Biochem, 1–7.
Sun, B., Ma, Y., Wang, F., Hu, L., & Sun, Y. (2019). miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther, 10(1), 360.
Yang, M., Lin, L., Sha, C., Li, T., Zhao, D., Wei, H., Chen, Q., Liu, Y., Chen, X., Xu, W., Li, Y., & Zhu, X. (2020). Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Laboratory Investigation, 100(3), 342–352.
Leopold, J. A. (2015). Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends in Cardiovascular Medicine, 25(4), 267–274.
Zhang, K., Zhang, Y., Feng, W., Chen, R., Chen, J., Touyz, R. M., Wang, J., & Huang, H. (2017). Interleukin-18 enhances vascular calcification and Osteogenic differentiation of vascular smooth muscle cells through TRPM7 activation. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(10), 1933–1943.
Wang, Y., Ma, W. Q., Zhu, Y., Han, X. Q., & Liu, N. (2018). Exosomes Derived From Mesenchymal Stromal Cells Pretreated With Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells. Front Endocrinol (Lausanne), 9, 524.
Ling, X., Zhang, G., Xia, Y., Zhu, Q., Zhang, J., Li, Q., Niu, X., Hu, G., Yang, Y., Wang, Y., & Deng, Z. (2020). Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. Journal of Cellular and Molecular Medicine, 24(1), 640–654.
Guo, Y., & Niu, S. (2018). MiR-25 protects PC-12 cells from H2O2 mediated oxidative damage via WNT/β-catenin pathway. The Journal of Spinal Cord Medicine, 41(4), 416–425.
Acknowledgements
All of the persons contributed to the manuscript was placed as an Author in the manuscript.
Availability of Data and Materials
In this ‘Review’ no publicly available datasets were used. All of the articles discussed were cited.
Funding
‘This work is not supported by any institution’.
Author information
Authors and Affiliations
Contributions
Prof. Dr. Zafer Cetin conducted literature surveys and prepared the main text, Fig. 1 and Table 1, Assist Prof. Gökhan Görgişen contributed the section 6, Assist. Prof. Emel Sokullu contributed to sections 2/3 and edited the manuscript, and Prof. Dr. Eyup Ilker Saygili contributed to the configuration of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
This manusript is only prepared for scientific purposes and ‘There is no conflict of interests’.
Consent for Publication
All authors of the manuscript; have read and agreed the Journal BioMed Central ‘Copyright and License Policy’, have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria and decleare that ‘The Article’ is original, has not already been published in a journal, and is not currently under consideration by another journal.
Ethics Approval and Consent to Participate
Not applicable.
Additional information
This article belongs to the Topical Collection: Special Issue on Exosomes and Microvesicles: from Stem Cell Biology to Translation in Human Diseases
Guest Editor: Giovanni Camussi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cetin, Z., Saygili, E.I., Görgisen, G. et al. Preclinical Experimental Applications of miRNA Loaded BMSC Extracellular Vesicles. Stem Cell Rev and Rep 17, 471–501 (2021). https://doi.org/10.1007/s12015-020-10082-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10082-x