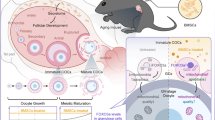
Abstract
Premature ovarian insufficiency (POI), a fertility disorder affecting women under 40 years of age, is characterized by early loss of ovarian function. This study was aimed to maintain ovarian function in POI animal models by mesenchymal stem cells (MSCs) transplantation with/without the supplementation of platelet-rich plasma (PRP). Adipose tissue-derived MSCs were isolated from inbred rats (Fisher-344), and constitutive expression of both VEGF and GFP were maintained by transfection with plasmids, pVEGF and pGFP-N. PRP was derived from the blood of healthy untreated rats. A total of 60 rats were divided into 5 groups of 12 rats in each. First group was kept as untreated-control (Control), and POI model was induced in Fisher-344 rats by cyclophosphamide in the next four groups. Second group was kept as sham-operated-control (Sham). MSC, PRP and MSC+ PRP-treated groups were transplanted following the validation of POI model in rats. After 2 months following the transplantation, anti-mullerian-hormone (AMH) and oestradiol (E2) blood levels were measured. Follicles were evaluated after hematoxylin-eosin staining, and the immunofluorescence staining and gene expression analyses were performed to show the ovarian regeneration. The follicular count was improved after MSC- and MSC + PRP-treatment to 63% of Control-group and significantly higher than that in Sham-group, but a significant increase was not observed in PRP-group. Higher AMH and E2 levels were measured in MSC + PRP than in Sham-group, and CXCL12, BMP-4, TGF-β and IGF-1 expressions were also increased. This study showed MSCs +/-PRP transplantation after POI supports recovery of the follicular count and function. For ovarian recovery, a single administration of PRP was found not sufficient. Although MSC treatment increased follicular regeneration, better results were obtained in the co-transplantation of MSCs and PRP. These results might be promising for follicular regeneration in POI patients.







Similar content being viewed by others
Abbreviations
- AMH:
-
Anti-mullerian-hormone
- AT-MSCs:
-
Adipose tissue-derived mesenchymal stem cells
- bFGF:
-
Basic fibroblast growth factor
- BMP-4:
-
Bone morphogenetic protein 4
- CNTF:
-
Ciliary neurotropic factor
- CXCL12:
-
C-X-C motif chemokine ligand 12
- CYP19A:
-
Cytochrome P450 aromatase
- R:
-
Pearson product-moment correlation coefficient
- E2 :
-
Oestradiol
- FBS:
-
Foetal bovine serum
- G3PDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GDF9:
-
Growth Differentiation factor 9
- GFP:
-
Green fluorescent protein
- GV:
-
Germinal vesicle
- H&E:
-
Hematoxylin and Eosin
- IGF-1:
-
Insulin-like growth factor 1
- IL-10:
-
Interleukin 10
- IL1b:
-
Interleukin 1b
- KGF:
-
Keratinocyte growth factor
- KL:
-
Kit-ligand
- LIF:
-
Leukemia inhibitory factor
- MSCs:
-
Mesenchymal stem cells
- OSCs:
-
Oogonial stem cells
- p:
-
Probability
- PCNA:
-
Proliferating cell nuclear antigen
- POI:
-
Premature ovarian insufficiency
- PRP:
-
Platelet-rich plasma
- TGF-β:
-
Transforming growth factor beta 1
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- VEGF:
-
Vascular endothelial growth factor
- VSEL:
-
Very small embryonic-like stem cells
References
Woad, K. J., Watkins, W. J., Prendergast, D., & Shelling, A. N. (2006). The genetic basis of premature ovarian failure. Australian and New Zealand Journal of Obstetrics and Gynaecology, 46, 242–244.
Shelling, A. N. (2010). Premature ovarian failure. Reproduction, 140, 633–641.
Sheikhansari, G., Aghebati-Maleki, L., Nouri, M., Jadidi-Niaragh, F., & Yousefi, M. (2018). Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomedicine & Pharmacotherapy, 102, 254–262.
Sukur, Y. E., Kivancli, I. B., & Ozmen, B. (2014). Ovarian aging and premature ovarian failure. Journal of The Turkish-German Gynecological Association, 15, 190–196.
Bedoschi, G., Navarro, P. A., & Oktay, K. (2016). Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncology, 2, 2333–2344.
Soleimani, R., Heytens, E., Darzynkiewicz, Z., & Oktay, K. (2011). Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging, 3, 782–793.
Zhou, L., Xie, Y., Li, S., et al. (2017). Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. Journal of Ovarian Research, 10(1), 56.
Yuksel, A., Bildik, G., Senbabaoglu, F., et al. (2015). The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Human Reproduction, 30, 2926–2935.
Galvez-Martin, P., Sabata, R., Verges, J., Zugaza, J. L., Ruiz, A., & Clares, B. (2016). Mesenchymal stem cells as therapeutics agents: Quality and environmental regulatory aspects. Stem Cells International, 2016, 9783408.
Qiu, P., Bai, Y., Pan, S., Li, W., Liu, W., & Hua, J. (2013). Gender depended potentiality of differentiation of human umbilical cord mesenchymal stem cells into oocyte-like cells in vitro. Cell Biochemistry and Function, 31, 365–373.
Bukovsky, A. (2011). Immune maintenance of self in morphostasis of distinct tissues, tumor growth, and regenerative medicine. Scandinavian Journal of Immunology, 73, 159–189.
Pourgholaminejad, A., Aghdami, N., Baharvand, H., & Moazzeni, S. M. (2016). The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine, 85, 51–60.
Caplan, A. I., & Correa, D. (2011). The MSC: An injury drugstore. Cell Stem Cell, 9, 11–15.
Gobbi, A., & Fishman, M. (2016). Platelet-rich plasma and bone marrow-derived mesenchymal stem cells in sports medicine. Sports Medicine and Arthroscopy Review, 24, 69–73.
Sills, E. S., Rickers, N. S., Li, X., & Palermo, G. D. (2018). First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecological Endocrinology, 2018, 1–5.
Wang, S., Yu, L., Sun, M., et al. (2013). The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. BioMed Research International, 2013, 690491.
Liu, T., Huang, Y., Guo, L., Cheng, W., & Zou, G. (2012). CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. International Journal of Medical Sciences, 9, 592–602.
Johnson, J., Bagley, J., Skaznik-Wikiel, M., et al. (2005). Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell, 122, 303–315.
Lee, H. J., Selesniemi, K., Niikura, Y., et al. (2007). Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. Journal of Clinical Oncology, 25, 3198–3204.
Santiquet, N., Vallières, L., Pothier, F., Sirard, M. A., Robert, C., & Richard, F. (2012). Transplanted bone marrow cells do not provide new oocytes but rescue fertility in female mice following treatment with chemotherapeutic agents. Cellular Reprogramming, 14, 123–129.
Wang, Z., Wang, Y., Yang, T., Li, J., & Yang, X. (2017). Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Research & Therapy, 8, 11.
Sun, M., Wang, S., Li, Y., et al. (2013). Adipose-derived stem cells improved mouse ovary function after hemotherapy-induced ovary failure. Stem Cell Research & Therapy, 4, 80.
Takehara, Y., Yabuuchi, A., Ezoe, K., et al. (2013). The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Laboratory Investigation, 93, 181–193.
Demirayak, B., Yüksel, N., Çelik, O. S., et al. (2016). Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Experimental Eye Research, 151, 227–235.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Kortenbach, I., Marini, F., & Krause, D. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Ozbek, E., Adas, G., Otunctemur, A., et al. (2015). Role of mesenchymal stem cells transfected with vascular endothelial growth factor in maintaining renal structure and function in rats with unilateral ureteral obstruction. Experimental and Clinical Transplantation, 13, 262–272.
Öksüz, S., Alagöz, M. Ş., Karagöz, H., et al. (2015). Comparison of treatments with local mesenchymal stem cells and mesenchymal stem cells with increased vascular endothelial growth factor expression on irradiation injury of expanded skin. Annals of Plastic Surgery, 75, 219–230.
Adas, G., Koc, B., Adas, M., et al. (2016). Effects of mesenchymal stem cells and VEGF on liver regeneration following major resection. Langenbeck's Archives of Surgery, 401, 725–740.
Nagata, M. J., Messora, M. R., Furlaneto, F. A., et al. (2010). Effectiveness of two methods for preparation of autologous platelet-rich plasma: An experimental study in rabbits. European Journal of Dentistry, 4, 395–402.
Cakici, C., Buyrukcu, B., Duruksu, G., et al. (2013). Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. BioMed Research International, 2013, 529589.
Sun, X., Su, Y., He, Y., et al. (2015). New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle, 14, 721–731.
Flaws, J. A., Abbud, R., Mann, R. J., et al. (1997). Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biological Reproduction, 57, 1233–1237.
Pedersen, T. (1970). Determination of follicle growth rate in the ovary of the immature mouse. Journal of Reproduction and Fertility, 21, 81–93.
Detre, S., Saclani Jotti, G., & Dowsett, M. A. J. (1995). A "quickscore" method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. Clinical Pathology, 48, 876–878.
Epstein, R. J. (1990). Drug-induced DNA damage and tumor chemosensitivity. Journal of Clinical Oncology, 8, 2062–2084.
Kilic, S., Pinarli, F., Ozogul, C., Tasdemir, N., Naz Sarac, G., & Delibasi, T. (2014). Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecological Endocrinology, 30, 135–140.
Song, D., Zhong, Y., Qian, C., et al. (2016). Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. BioMed Research International, 2016, 2517514.
Kerr, J. B., Brogan, L., Myers, M., et al. (2012). The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction, 143, 469–476.
Lei, L., & Spradling, A. C. (2013). Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proceedings of the National Academy of Sciences, 110, 8585–8590.
Yuan, J., Zhang, D., Wang, L., et al. (2013). No evidence for neo-oogenesis may link to ovarian senescence in adult monkey. Stem Cells, 31, 2538–2550.
Virant-Klun, I., Skutella, T., Bhartiya, D., & Jin, X. (2013). Stem cells in reproductive tissues: From the basics to clinics. BioMed Research International, 2013, 357102.
White, Y. A., Woods, D. C., Takai, Y., Ishihara, O., Seki, H., & Tilly, J. L. (2012). Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nature Medicine, 18, 413–421.
Johnson, J., Canning, J., Kaneko, T., Pru, J. K., & Tilly, J. L. (2004). Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature, 428, 145–150.
Bukovsky, A., Svetlikova, M., & Caudle, M. R. (2005). Oogenesis in cultures derived from adult human ovaries. Reproductive Biology and Endocrinology, 3, 17.
Bukovsky, A. (2011). Ovarian stem cell niche and follicular renewal in mammals. The Anatomical Record, 294, 1284–1306.
Bukovsky, A. (2015). Novel methods of treating ovarian infertility in older and POF women, testicular infertility, and other human functional diseases. Reproductive Biology and Endocrinology, 13, 10.
Hayama, T., Yamaguchi, T., Kato-Itoh, M., et al. (2014). Generation of mouse functional oocytes in rat by xeno-ectopic transplantation of primordial germ cells. Biology of Reproduction, 91, 89.
Morgan, S., Anderson, R. A., Gourley, C., Wallace, W. H., & Spears, N. (2012). How do chemotherapeutic agents damage the ovary? Human Reproduction Update, 18, 525–535.
Young, J. M., & McNeilly, A. S. (2010). Theca: The forgotten cell of the ovarian follicle. Reproduction, 140, 489–504.
Virant-Klun, I. (2015). Postnatal oogenesis in humans: A review of recent findings. Stem Cells Cloning, 8, 49–60.
Eppig, J. J. (2001). Oocyte control of ovarian follicular development and function in mammals. Reproduction, 122, 829–838.
Bukovsky, A., & Caudle, M. R. (2012). Immunoregulation of follicular renewal, selection, POF, and menopause in vivo, vs. neo-oogenesis in vitro, POF and ovarian infertility treatment and a clinical trial. Reproductive Biology and Endocrinology, 10, 97.
Frese, L., Dijkman, P. E., & Hoerstrup, S. P. (2016). Adipose tissue-derived stem cells in regenerative medicine. Transfusion Medicine and Hemotherapy, 43, 268–274.
Dewailly, D., Robin, G., Peigne, M., Decanter, C., Pigny, P., & Catteau-Jonard, S. (2016). Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Human Reproduction Update, 22, 709–724.
Virant-Klun, I., Skutella, T., Stimpfel, M., & Sinkovec, J. (2011). Ovarian surface epithelium in patients with severe ovarian infertility: A potential source of cells expressing markers of pluripotent/multipotent stem cells. Journal of Biomedicine and Biotechnology, 2011, 381928.
Huda, F., Fan, Y., Suzuki, M., et al. (2016). Fusion of human fetal mesenchymal stem cells with "degenerating" cerebellar neurons in spinocerebellar Ataxia type 1 model mice. PLoS One, 11, e0164202.
Binelli, M., & Murphy, B. D. (2010). Coordinated regulation of follicle development by germ and somatic cells. Reproduction Fertility and Development, 22(1), 12.
Shaikh, A., Anand, S., Kapoor, S., Ganguly, R., & Bhartiya, D. (2017). Mouse bone marrow VSELs exhibit differentiation into three embryonic germ lineages and Germ & Hematopoietic Cells in culture. Stem Cell Reviews and Reports, 13, 202–216.
Xia, X., Wang, T., Yin, T., Yan, L., Yan, J., & Lu, C. (2015). Mesenchymal stem cells facilitate in vitro development of human Preantral follicle. Reproductive Sciences, 22, 1367–1376.
Pangas, S. A. (2012). Regulation of the ovarian reserve by members of the transforming growth factor beta family. Molecular Reproduction and Development, 79, 666–679.
Chen, Y. C., Chang, H. M., Cheng, J. C., Tsai, H. D., Wu, C. H., & Leung, P. C. (2015). Transforming growth factor-β1 up-regulates connexin43 expression in human granulosa cells. Human Reproduction, 30, 2190–2201.
Al-Samerria, S., Al-Ali, I., McFarlane, J. R., & Almahbobi, G. (2015). The impact of passive immunisation against BMPRIB and BMP4 on follicle development and ovulation in mice. Reproduction, 149, 403–411.
Dunlop, C. E., & Anderson, R. A. (2014). The regulation and assessment of follicular growth. Scandinavian Journal of Clinical and Laboratory Investigation, 244, 13–17.
Park, E. S., Woods, D. C., & Tilly, J. L. (2013). Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertility and Sterility, 100, 1468–1475.
Bayne, R. A., Donnachie, D. J., Kinnell, H. L., Childs, A. J., & Anderson, R. A. (2016). BMP signalling in human fetal ovary somatic cells is modulated in a gene-specific fashion by GREM1 and GREM2. Molecular Human Reproduction, 22, 622–633.
Tan, S., Feng, B., Yin, M., et al. (2017). Stromal Senp1promotes mouse early folliculogenesis by regulating BMP4 expression. Cell & Bioscience, 7, 36.
Polat, I. M., Alçiğir, E., Pekcan, M., et al. (2015). Characterization of transforming growth factor beta superfamily, growth factors, transcriptional factors, and lipopolysaccharide in bovine cystic ovarian follicles. Theriogenology, 84, 1043–1052.
Vural, F., Vural, B., Doğer, E., Çakıroğlu, Y., & Çekmen, M. (2016). Perifollicular blood flow and its relationship with endometrial vascularity, follicular fluid EG-VEGF, IGF-1, and inhibin-a levels and IVF outcomes. Journal of Assisted Reproduction and Genetics, 33, 1355–1362.
Bukovsky, A., Keenan, J. A., Caudle, M. R., Wimalasena, J., Upadhyaya, N. B., & Van Meter, S. E. (1995). Immunohistochemical studies of the adult human ovary: Possible contribution of immune and epithelial factors to folliculogenesis. American Journal of Reproductive Immunology, 33, 323–340.
Jasti, S., Warren, B. D., McGinnis, L. K., Kinsey, W. H., Petroff, B. K., & Petroff, M. G. (2012). The autoimmune regulator prevents premature reproductive senescence in female mice. Biology of Reproduction, 86, 110.
Ye, H., Zheng, T., Li, W., et al. (2017). Ovarian stem cell nests in reproduction and ovarian aging. Cellular Physiology and Biochemistry, 43, 1917–1925.
Acknowledgements
We thank Experimental Medicine Research and Application Unit (DETAB) at Kocaeli University for their assistance in our experimental work. This work was supported by the Scientific and Research Council of Turkey (TUBITAK) [grant number 114S398].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of Interest
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vural, B., Duruksu, G., Vural, F. et al. Effects of VEGF + Mesenchymal Stem Cells and Platelet-Rich Plasma on Inbred Rat Ovarian Functions in Cyclophosphamide-Induced Premature Ovarian Insufficiency Model . Stem Cell Rev and Rep 15, 558–573 (2019). https://doi.org/10.1007/s12015-019-09892-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09892-5