Abstract
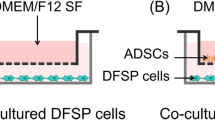
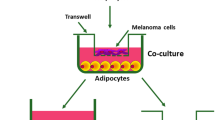
This study focuses on the interactions of human adipose tissue-derived stem cells (ADSCs) and malignant melanoma cells (MMCs) with regard to future cell-based skin therapies. The aim was to identify potential oncological risks as ADSCs could unintentionally be sited within the proximity of the tumor microenvironment of MMCs. An indirect co-culture model was used to analyze interactions between ADSCs and four different established melanoma cell lines (G-361, SK-Mel-5, MeWo and A2058) as well as two low-passage primary melanoma cell cultures (M1 and M2). Doubling time, migration and invasion, angiogenesis, quantitative real-time PCR of 229 tumor-associated genes and multiplex protein assays of 20 chemokines and growth factors and eight matrix metalloproteinases (MMPs) were evaluated. Co-culture with ADSCs significantly increased migration capacity of G-361, SK-Mel-5, A2058, MeWo and M1 and invasion capacity of G-361, SK-Mel-5 and A2058 melanoma cells. Furthermore, conditioned media from all ADSC-MMC-co-cultures induced tube formation in an angiogenesis assay in vitro. Gene expression analysis of ADSCs and MMCs, especially of low-passage melanoma cell cultures, revealed an increased expression of various genes with tumor-promoting activities, such as CXCL12, PTGS2, IL-6, and HGF upon ADSC-MMC-co-culture. In this context, a significant increase (up to 5,145-fold) in the expression of numerous tumor-associated proteins could be observed, e.g. several pro-angiogenic factors, such as VEGF, IL-8, and CCL2, as well as different matrix metalloproteinases, especially MMP-2. In conclusion, the current report clearly demonstrates that a bi-directional crosstalk between ADSCs and melanoma cells can enhance different malignant properties of melanoma cells in vitro.




Similar content being viewed by others
Abbreviations
- ADSCs:
-
Adipose tissue-derived stem cells
- bFGF:
-
Basic fibroblast growth factor
- CCL:
-
C-C motif-ligand
- CD:
-
Cluster of differentiation
- COX-2:
-
Cyclooxygenase-2
- CXCL:
-
C-X-C motif ligand
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial-mesenchymal-transition
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- FCS:
-
Fetal calf serum
- FN:
-
Fibronectin
- HGF:
-
Hepatocyte growth factor
- hMSCs:
-
Human mesenchymal stem/stroma cells
- HUVEC:
-
Human umbilical vein endothelial cells
- IL:
-
Interleukin
- MCAM:
-
Melanoma cell adhesion molecule
- MMCs:
-
Malignant melanoma cells
- MMP:
-
Matrix metalloproteinase
- PMA:
-
Phorbol 12-myristate 13-acetate
- SD:
-
Standard deviation
- PTGS2:
-
Prostaglandin-endoperoxide synthase 2
- TW:
-
Transwell
- VEGF:
-
Vascular endothelial growth factor.
References
Kapur, S. K., & Katz, A. J. (2013). Review of the adipose derived stem cell secretome. Biochimie, 95, 2222–2228.
Gimble, J., & Guilak, F. (2003). Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy, 5, 362–369.
Alperovich, M., Lee, Z. H., Friedlander, P. L., Rowan, B. G., Gimble, J. M., & Chiu, E. S. (2014). Adipose stem cell therapy in cancer reconstruction: a critical review. Annals of Plastic Surgery, 73(Suppl 1), S104-7.
Sterodimas, A., de Faria, J., Nicaretta, B., & Pitanguy, I. (2010). Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS, 63, 1886–1892.
Rodriguez, J., Boucher, F., Lequeux, C., et al. (2015). Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Research & Therapy, 6, 241.
Li, Y., Zhang, W., Gao, J., et al. (2016). Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Research & Therapy, 7, 102.
Kim, W. S., Park, B. S., Park, S. H., Kim, H. K., & Sung, J. H. (2009). Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. Journal of Dermatological Science, 53, 96–102.
Kim, W. S., Park, B. S., & Sung, J. H. (2009). The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opinion Biological Therapy, 9, 879 – 87.
Kim, W. S., Park, B. S., & Sung, J. H. (2009). Protective role of adipose-derived stem cells and their soluble factors in photoaging. Archives of Dermatological Research, 301:329 – 36.
Kim, W. S., Park, S. H., Ahn, S. J., et al. (2008). Whitening effect of adipose-derived stem cells: a critical role of TGF-beta 1. Biological & Pharmaceutical Bulletin, 31:606 – 10.
Kim, J. H., Jung, M., Kim, H. S., Kim, Y. M., & Choi, E. H. (2011). Adipose-derived stem cells as a new therapeutic modality for ageing skin. Experimental Dermatology, 20, 383–387.
Jackson, W. M., Nesti, L. J., & Tuan, R. S. (2012). Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Research & Therapy, 3, 20.
Lin, G., Yang, R., Banie, L., et al. (2010). Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. The Prostate, 70, 1066–1073.
Jotzu, C., Alt, E., Welte, G., et al. (2010). Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Analytical Cellular Pathology (Amsterdam), 33, 61–79.
Koellensperger, E., Gramley, F., Preisner, F., Leimer, U., Germann, G., & Dexheimer, V. (2014). Alterations of gene expression and protein synthesis in co-cultured adipose tissue-derived stem cells and squamous cell-carcinoma cells: consequences for clinical applications. Stem Cell Research & Therapy, 5, 65.
Kucerova, L., Matuskova, M., Hlubinova, K., Altanerova, V., & Altaner, C. (2010). Tumor cell behaviour modulation by mesenchymal stromal cells. Molecular Cancer, 9, 129.
Karnoub, A. E., Dash, A. B., Vo, A. P., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449, 557 – 63.
Donnenberg, V. S., Zimmerlin, L., Rubin, J. P., Donnenberg, A. D.. (2010). Regenerative therapy after cancer: what are the risks? Tissue engineering Part B. Reviews, 16:567 – 75.
Chen, S. T., Geller, A. C., & Tsao, H. (2013). Update on the epidemiology of melanoma. Current Dermatology Reports, 2, 24–34.
Nikolaou, V., & Stratigos, A. J. (2014). Emerging trends in the epidemiology of melanoma. The British Journal of Dermatology, 170:11 – 9.
Koellensperger, E., Bollinger, N., Dexheimer, V., Gramley, F., Germann, G., & Leimer, U. (2014). Choosing the right type of serum for different applications of human adipose tissue-derived stem cells: influence on proliferation and differentiation abilities. Cytotherapy, 16, 789 – 99.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International society for cellular therapy position statement. Cytotherapy, 8, 315–317.
Bourin, P., Bunnell, B. A., Casteilla, L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15, 641–648.
Lindroos, B., Suuronen, R., & Miettinen, S. (2011). The potential of adipose stem cells in regenerative medicine. Stem Cell Reviews, 7, 269 – 91.
Kim, W. S., Park, B. S., Sung, J. H., et al. (2007). Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. Journal of Dermatological Science, 48, 15–24.
Choi, E. W., Shin, I. S., Song, J. W., et al. (2015). Transplantation of adipose tissue-derived mesenchymal stem cells prevents the development of Lupus Dermatitis. Stem Cells and Development, 24, 2041–2051.
Reichenberger, M. A., Heimer, S., Schaefer, A., et al. (2012). Adipose derived stem cells protect skin flaps against ischemia-reperfusion injury. Stem Cell Reviews, 8, 854 – 62.
Prantl, L., Muehlberg, F., Navone, N. M., et al. (2010). Adipose tissue-derived stem cells promote prostate tumor growth. The Prostate, 70, 1709–1715.
Jotzu, C., Alt, E., Welte, G., et al. (2011). Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cellular Oncology, 34, 55–67.
Devarajan, E., Song, Y. H., Krishnappa, S., & Alt, E. (2012). Epithelial-mesenchymal transition in breast cancer lines is mediated through PDGF-D released by tissue-resident stem cells. International Journal of Cancer Journal International du Cancer, 131, 1023–1031.
Kamat, P., Schweizer, R., Kaenel, P., et al. (2015). Human adipose-derived mesenchymal stromal cells may promote breast cancer progression and metastatic spread. Plastic and Reconstructive Surgery, 136, 76–84.
Lee, J. H., Park, C. H., Chun, K. H., & Hong, S. S. (2015). Effect of adipose-derived stem cell-conditioned medium on the proliferation and migration of B16 melanoma cells. Oncology Letters, 10, 730–736.
Ahn, J. O., Coh, Y. R., Lee, H. W., Shin, I. S., Kang, S. K., & Youn, H. Y. (2015). Human adipose tissue-derived mesenchymal stem cells inhibit melanoma growth in vitro and in vivo. Anticancer Research, 35, 159 – 68.
Bahrambeigi, V., Ahmadi, N., Salehi, R., & Javanmard, S. H. (2015). Genetically modified murine adipose-derived mesenchymal stem cells producing interleukin-2 favor B16F10 melanoma cell proliferation. Immunological Investigations, 44, 216 – 36.
Esquenet, M., Swinnen, J. V., Heyns, W., & Verhoeven, G. (1997). LNCaP prostatic adenocarcinoma cells derived from low and high passage numbers display divergent responses not only to androgens but also to retinoids. The Journal of Steroid Biochemistry and Molecular Biology, 62, 391–399.
Lin, H. K., Hu, Y. C., Yang, L., et al. (2003). Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. The Journal of Biological Chemistry, 278, 50902–50907.
O’Driscoll, L., Gammell, P., McKiernan, E., et al. (2006). Phenotypic and global gene expression profile changes between low passage and high passage MIN-6 cells. The Journal of Endocrinology, 191, 665 – 76.
Neumann, E., Riepl, B., Knedla, A., et al. (2010). Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Research & Therapy, 12, R83.
Hughes, P., Marshall, D., Reid, Y., Parkes, H., & Gelber, C. (2007). The costs of using unauthenticated, over-passaged cell lines: how much more data do we. need? Biotechniques, 43(575), 7–8.
Mouriaux, F., Zaniolo, K., Bergeron, M. A., et al. (2016). Effects of long-term serial passaging on the characteristics and properties of cell lines derived from uveal melanoma primary tumors. Investigative Ophthalmology and Visual Science, 57, 5288 – 301.
Halaban, R., Rubin, J. S., Funasaka, Y., et al. (1992). Met and hepatocyte growth factor/scatter factor signal transduction in normal melanocytes and melanoma cells. Oncogene, 7, 2195 – 206.
Natali, P. G., Nicotra, M. R., Di Renzo, M. F., et al. (1993). Expression of the c-Met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. British Journal of Cancer; 68:746 – 50.
Otsuka, T., Takayama, H., Sharp, R., et al. (1998). c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Research, 58, 5157–5167.
Recio, J. A., & Merlino, G. (2002). Hepatocyte growth factor/scatter factor activates proliferation in melanoma cells through p38 MAPK, ATF-2 and cyclin D1. Oncogene, 21, 1000–1008.
Aggarwal, B. B., Sethi, G., Ahn, K. S., et al. (2006). Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Annals of the New York Academy of Sciences, 1091, 151–169.
Di, G. H., Liu, Y., Lu, Y., Liu, J., Wu, C., & Duan, H. F. (2014). IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PloS one, 9, e113572.
Li, J., Lan, T., Zhang, C., et al. (2015). Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget, 6, 1031–1048.
Scherzad, A., Steber, M., Gehrke, T., et al. (2015). Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. International Journal of Oncology, 47, 391–397.
Wei, H. J., Zeng, R., Lu, J. H., et al. (2015). Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget, 6, 7713–7726.
Lu, C., & Kerbel, R. S. (1993). Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. The Journal of Cell Biology, 120, 1281–1288.
Lu, C., Vickers, M. F., & Kerbel, R. S. (1992). Interleukin 6: a fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Proceedings of the National Academy of Sciences of the United States of America, 89:9215-9.
Seymour, J. F., Talpaz, M., Cabanillas, F., Wetzler, M., & Kurzrock, R. (1995). Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 13, 575 – 82.
Chang, P. H., Pan, Y. P., Fan, C. W., et al. (2016). Pretreatment serum interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha levels predict the progression of colorectal cancer. Cancer Medicine, 5, 426 – 33.
Nakashima, J., Tachibana, M., Horiguchi, Y., et al. (2000). Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 6, 2702–2706.
Kim, D. K., Oh, S. Y., Kwon, H. C., et al. (2009). Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer, 9, 155.
Hoejberg, L., Bastholt, L., Johansen, J. S., Christensen, I. J., Gehl, J., & Schmidt, H. (2012). Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Research, 22, 287 – 93.
Carmeliet, P. (2005). VEGF as a key mediator of angiogenesis in cancer. Oncology, 69(Suppl 3), 4–10.
Bar-Eli, M. (1999). Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology: Journal of Immunopathology, Molecular and Cellular Biology, 67:12 – 8.
Koch, A. E., Polverini, P. J., Kunkel, S. L., et al. (1992). Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science, 258, 1798 – 801.
Cho, H. H., Kim, Y. J., Kim, J. T., et al. (2009). The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 24, 511–518.
Van Coillie, E., Van Aelst, I., Wuyts, A., et al. (2001). Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. The American Journal of Pathology, 159, 1405–1414.
Gazzaniga, S., Bravo, A. I., Guglielmotti, A., et al. (2007). Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. The Journal of Investigative Dermatology, 127, 2031–2041.
Koga, M., Kai, H., Egami, K., et al. (2008),nt melanoma in mice. Biochemical and Biophysical Research Communications, 365:279 – 84.
Wu, S., Singh, S., Varney, M. L., Kindle, S., & Singh, R. K. (2012). Modulation of CXCL-8 expression in human melanoma cells regulates tumor growth, angiogenesis, invasion, and metastasis. Cancer Medicine, 1, 306 – 17.
Li, A., Dubey, S., Varney, M. L., Dave, B. J., & Singh, R. K. (2003). IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. Journal of Immunology, 170, 3369–3376.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Luca, M., Huang, S., Gershenwald, J. E., Singh, R. K., Reich, R., & Bar-Eli, M. (1997). Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. The American Journal of Pathology, 151, 1105–1113.
Hadler-Olsen, E., Fadnes, B., Sylte, I., Uhlin-Hansen, L., & Winberg, J. O. (2011). Regulation of matrix metalloproteinase activity in health and disease. The FEBS Journal, 278, 28–45.
Valente, P., Fassina, G., Melchiori, A., et al. (1998). TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. International Journal of Cancer Journal International du Cancer, 75, 246 – 53.
Miyoshi, A., Kitajima, Y., Kido, S., et al. (2005). Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. British Journal of Cancer, 92, 252–258.
Reis, S. T., Leite, K. R., Piovesan, L. F., et al. (2012). Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urology, 12, 18.
Wang, Y. Z., Wu, K. P., Wu, A. B., et al. (2014). MMP-14 overexpression correlates with poor prognosis in non-small cell lung cancer. Tumour biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine, 35, 9815–9821.
Zhao, S., Ma, W., Zhang, M., et al. (2013). High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Medical Oncology, 30, 335.
Hofmann, U. B., Eggert, A. A., Blass, K., Brocker, E. B., & Becker, J. C. (2005). Stromal cells as the major source for matrix metalloproteinase-2 in cutaneous melanoma. Archives of Dermatological Research, 297:154 – 60.
Kanekura, T., & Chen, X. (2010). CD147/basigin promotes progression of malignant melanoma and other cancers. Journal of dermatological science ;57:149 – 54.
Hatanaka, M., Higashi, Y., Fukushige, T., et al. (2014). Cleaved CD147 shed from the surface of malignant melanoma cells activates MMP2 produced by fibroblasts. Anticancer Research, 34, 7091–7096.
Tang, Y., Nakada, M. T., Rafferty, P., et al. (2006). Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Molecular Cancer Research, MCR, 4, 371–377.
Kanekura, T., Chen, X., & Kanzaki, T. (2002). Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. International Journal of Cancer Journal International du Cancer, 99, 520–528.
Alonso, S. R., Tracey, L., Ortiz, P., et al. (2007). A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Research, 67, 3450–3460.
Hao, L., Ha, J. R., Kuzel, P., Garcia, E., & Persad, S. (2012). Cadherin switch from E- to N-cadherin in melanoma progression is regulated by the PI3K/PTEN pathway through Twist and Snail. The British Journal of Dermatology, 166, 1184–1197.
Demaria, S., Pikarsky, E., Karin, M., et al. (2010). Cancer and inflammation: promise for biologic therapy. Journal of Immunotherapy, 33, 335–351.
Fujimoto, H., Sangai, T., Ishii, G., et al. (2009). Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. International Journal of Cancer Journal International du Cancer, 125, 1276–1284.
Ohta, M., Kitadai, Y., Tanaka, S., et al. (2002). Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. International Journal of Cancer Journal International du Cancer, 102, 220–224.
Ohta, M., Kitadai, Y., Tanaka, S., et al. (2003). Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. International Journal of Oncology, 22, 773–778.
Ueno, T., Toi, M., Saji, H., et al. (2000). Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clinical Cancer Research : An official journal of the American Association for Cancer Research, 6, 3282–3289.
Mitchell, B., & Mahalingam, M. (2014). The CXCR4/CXCL12 axis in cutaneous malignancies with an emphasis on melanoma. Histology and Histopathology, 29, 1539–1546.
Sun, X., Cheng, G., Hao, M., et al. (2010) CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Reviews, 29:709 – 22.
Mortier, A., Gouwy, M., Van Damme, J., & Proost, P. (2011). Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Experimental Cell Research, 317, 642 – 54.
Eferl, R., & Wagner, E. F. (2003). AP-1: a double-edged sword in tumorigenesis. Nature Reviews Cancer 3:859 – 68.
Kappelmann, M., Bosserhoff, A., & Kuphal, S. (2014). AP-1/c-Jun transcription factors: regulation and function in malignant melanoma. European Journal of Cell Biology, 93, 76–81.
Shu, W., Shu, Y. T., Dai, C. Y., & Zhen, Q. Z. (2012). Comparing the biological characteristics of adipose tissue-derived stem cells of different persons. Journal of Cellular Biochemistry, 113, 2020–2026.
Shain, A. H., Yeh, I., Kovalyshyn, I., et al. (2015). The genetic evolution of melanoma from precursor lesions. The New England Journal of Medicine, 373, 1926–1936.
Vizkeleti, L., Kiss, T., Koroknai, V., et al. (2017). Altered integrin expression patterns shown by microarray in human cutaneous melanoma. Melanoma Research, 27, 180–188.
Riker, A. I., Enkemann, S. A., Fodstad, O., et al. (2008). The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Medical Genomics, 1, 13.
Koh, S. S., Wei, J. P., Li, X., et al. (2012). Differential gene expression profiling of primary cutaneous melanoma and sentinel lymph node metastases. Modern pathology : An Official Journal of the United States and Canadian Academy of Pathology, Inc, 25, 828 – 37.
Kandarakov, O. F., Kopantseva, E. E., & Belyavsky, A. V. (2016). Analysis of proliferation of melanoma cells and mesenchymal stem cells in co-culture and contribution of experimental conditions into interpretation of the results. Bulletin of Experimental Biology and Medicine 162:127 – 33.
Acknowledgements
We would like to thank Claudia Ziegelmeier and Iris Kaiser for technical support with the multiplex analysis and Prof. Dr. Holger Sültmann (Division of Cancer Genome Research, NCT and German Cancer Research Center, Heidelberg, Germany) for providing access to the Bio-Plex 200 System.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Preisner, F., Leimer, U., Sandmann, S. et al. Impact of Human Adipose Tissue-Derived Stem Cells on Malignant Melanoma Cells in An In Vitro Co-culture Model. Stem Cell Rev and Rep 14, 125–140 (2018). https://doi.org/10.1007/s12015-017-9772-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9772-y