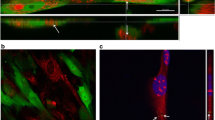
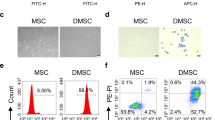
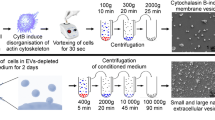
Abstract
Strategies that induce immunogenic cell death (ICD) or downregulate CD47 or PD-L1 expression have resulted in successful therapeutic options for tumor eradication. Several groups have reported the tumoricidal effects of human umbilical cord Wharton’s jelly stem cells (hWJSCs) or its conditioned medium (hWJSC-CM) on certain cancers but the mechanisms have not been elucidated. Since hWJSCs possess immunomodulatory properties, we investigated whether one of the tumoricidal mechanisms was via ICD. We first concentrated hWJSC-CM into a 3 kDa concentrate and then exposed various concentrations of this concentrate to human lymphoma cells to find out which concentration had the greatest tumoricidal effect. We observed that a 500 µg/ml concentration of the concentrate had the greatest inhibitory effect. Thereafter, lymphoma cells were exposed to 500 µg/ml of the hWJSC-CM-3 kDa concentrate and then subjected to analysis for morphology, viability, apoptosis, mitochondrial and endoplasmic reticulum stress, danger associated molecular patterns (DAMP), extracellular HMGB1, CD47 and PD-L1 markers and dendritic cell phenotype. Extensive nuclear chromatin and mitochondrial changes with significantly decreased cell viability and increased apoptosis were observed in the treated lymphoma cells compared to controls. There were also significant increases in the release of DAMPs, extracellular HMGB1 and dendritic cell activation and maturation, with concomitant decreases in CD47 and PD-L1 expression in the treated cells compared to controls. In other ongoing studies we observed increased expression of specific tumor-suppressor molecules (miRNA-146a and miRNA-126, MCP-1, IL-6, IL-8 and IL-12) in hWJSC-CM suggesting that one or more of these molecules may be the modulators of the ICD.







Similar content being viewed by others
References
Bracci, L., Schiavoni, G., Sistigu, A., et al. (2014). Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death and Differentiation, 21, 15–25.
Inoue, H., & Tani, K. (2014). Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death and Differentiation, 21, 39–49.
Martins, I., Wang, Y., Michaud, M., et al. (2014). Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death and Differentiation, 21, 79–91.
Kroemer, G., Galluzzi, L., Kepp, O., et al. (2013). Immunogenic cell death in cancer therapy. Annual Review of Immunology, 31, 51–72.
Chatterjee, K., Zhang, J., Honbo, N., et al. (2010). Doxorubicin cardiomyopathy. Cardiology, 115, 155–162.
Thompson, C. B. (1995). Apoptosis in the pathogenesis and treatment of disease. Science, 267, 1456–1462.
Igney, F. H., & Krammer, P. H. (2002). Death and anti-death: tumour resistance to apoptosis. Nature Reviews Cancer, 2, 277–288.
Kono, K., Mimura, K., & Kiessling, R. (2013). Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death & Disease, 4, e688.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy, 8, 315–317.
Fong, C. Y., Subramanian, A., Biswas, A., et al. (2010). Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human wharton’s jelly stem cells. Reproductive Biomedicine Online, 21, 391–401.
Troyer, D. L., & Weiss, M. L. (2008). Concise review: wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells, 26, 591–599.
Anzalone, R., Lo Iacono, M., Loria, T., et al. (2011) Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cells Revs and Reports, 7:342 – 63.
Bongso, A., & Fong, C. Y. (2013). The therapeutic potential, challenges and future clinical directions of stem cells from the wharton’s jelly of the human umbilical cord. Stem Cell Reviews, 9, 226–240.
Subramanian, A., Shu-Uin, G., Kae-Siang, N., et al. (2012). Human umbilical cord wharton’s jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. Journal of Cellular Biochemistry, 113, 1886–1895.
Chen, H., Zhang, N., Li, T., et al. (2012). Human umbilical cord wharton’s jelly stem cells: immune property genes assay and effect of transplantation on the immune cells of heart failure patients. Cellular Immunology, 276, 83–90.
Gauthaman, K., Fong, C. Y., Suganya, C. A., et al. (2012). Extra-embryonic human wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reproductive Biomedicine Online, 24, 235–246.
Wang, Y., Han, Z. B., Ma, J., et al. (2012). A toxicity study of multiple-administration human umbilical cord mesenchymal stem cells in cynomolgus monkeys. Stem Cells and Development, 21, 1401–1408.
Wu, K. H., Sheu, J. N., Wu, H. P., et al. (2013). Cotransplantation of umbilical cord-derived mesenchymal stem cells promote hematopoietic engraftment in cord blood transplantation: a pilot study. Transplantation, 95, 773–777.
Fan, C. G., Zhang, Q. J., & Zhou, J. R. (2011). Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Reviews, 7, 195–207.
Chao, Y. H., Tsai, C., Peng, C. T., et al. (2011). Cotransplantation of umbilical cord MSCs to enhance engraftment of hematopoietic stem cells in patients with severe aplastic anemia. Bone Marrow Transplantation, 46, 1391–1392.
Hu, J., Yu, X., Wang, Z., et al. (2013). Long term effects of the implantation of wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocrine Journal, 60, 347–357.
Dalous, J., Larghero, J., & Baud, O. (2012). Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatric Research, 71, 482–490.
Wang, X. Y., Lan, Y., He, W. Y., et al. (2008). Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood, 111, 2436–2443.
Gauthaman, K., Fong, C. Y., Cheyyatraivendran, S., et al. (2012). Human umbilical cord wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. Journal of Cellular Biochemistry, 113, 2027–2039.
Gauthaman, K., Fong, C. Y., Arularasu, S., et al. (2013). Human wharton’s jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth in vitro and in xenograft mice. Journal of Cellular Biochemistry, 114, 366–377.
Rachakatla, R. S., Marini, F., Weiss, M. L., et al. (2007). Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Therapy, 14, 828–835.
Ayuzawa, R., Doi, C., Rachakatla, R. S., et al. (2009). Naive human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Letters, 280, 31–37.
Tamura, M., Kawabata, A., Ohta, N., et al. (2011). Wharton’s jelly stem cells as agents for cancer therapy. The Open Tissue Engineering and Regenerative Medicine Journal, 4, 39–47.
Maurya, D. K., Doi, C., Kawabata, A., et al. (2010). Therapy with un-engineered naive rat umbilical cord matrix stem cells markedly inhibits growth of murine lung adenocarcinoma. BMC Cancer, 10, 590.
Chao, K. C., Yang, H. T., & Chen, M. W. (2012). Human umbilical cord mesenchymal stem cells suppress breast cancer tumourigenesis through direct cell-cell contact and internalization. Journal of Cellular and Molecular Medicine, 16, 1803–1815.
Liu, J., Han, G., Liu, H., et al. (2013). Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PloS One, 8, e62844.
Ma, Y., Hao, X., Zhang, S., et al. (2012). The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Research and Treatment, 133, 473–485.
Wu, S., Ju, G. Q., Du, T., et al. (2013). Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PloS One, 8, e61366.
Lin, H. D., Fong, C. Y., Biswas, A., et al. (2014). Human Wharton’s jelly stem cells, its conditioned medium and cell-free lysate inhibit the growth of human lymphoma cells. Stem Cell Reviews and Reports, 10, 573–586.
Kawabata, A., Ohta, N., Seiler, G., et al. (2013). Naive rat umbilical cord matrix stem cells significantly attenuate mammary tumor growth through modulation of endogenous immune responses. Cytotherapy, 15, 586–597.
Yang, H. T., & Chao, K. C. (2013). Foetal defence against cancer: a hypothesis. Journal of Cellular and Molecular Medicine, 17, 1096–1098.
Potter, J. F., & Schoeneman, M. (1970). Metastasis of maternal cancer to the placenta and fetus. Cancer, 25, 380–388.
Dildy, G. A., Moise, K. J. Jr., Carpenter, R. J. Jr., et al. (1989). Maternal malignancy metastatic to the products of conception: a review. Obstetrical & Gynecological Survey, 44, 535–540.
Alexander, A., Samlowski, W. E., Grossman, D., et al. (2003). Metastatic melanoma in pregnancy: risk of transplacental metastases in the infant. Journal of Clinical Oncology: Official journal of the American Society of Clinical Oncology, 21, 2179–2186.
Jackisch, C., Louwen, F., Schwenkhagen, A., et al. (2003). Lung cancer during pregnancy involving the products of conception and a review of the literature. Archives of Gynecology and Obstetrics, 268, 69–77.
Liu, J., & Guo, L. (2006). Intraplacental choriocarcinoma in a term placenta with both maternal and infantile metastases: a case report and review of the literature. Gynecologic Oncology, 103, 1147–1151.
Weiss, M. L., Medicetty, S., Bledsoe, A. R., et al. (2006). Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells, 24, 781–792.
Tipnis, S., Viswanathan, C., & Majumdar, A. S. (2010). Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunology and Cell Biology, 88, 795–806.
Barcia, R. N., Santos, J. M., Filipe, M., et al. (2015). What makes umbilical cord tissue-derived mesenchymal stromal cells superior immunomodulators when compared to bone marrow derived mesenchymal stromal cells? Stem Cells International, 2015:583984.
Fong, C. Y., Gauthaman, K., Cheyyatraivendran, S., et al (2012). Human umbilical cord Wharton’s jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. Journal of Cellular Biochemistry, 113, 658–668.
Sze, S. K., de Kleijn, D. P., Lai, R. C., et al. (2007). Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular & Cellular Proteomics: MCP, 6, 1680–1689.
Pereira, T., Armada-da Silva, P. A., Amorim, I., et al. (2014). Effects of human mesenchymal stem cells isolated from Wharton’s jelly of the umbilical cord and conditioned media on skeletal muscle regeneration using a myectomy model. Stem Cells International, 2014:376918.
Dangkong, D., & Limpanasithikul, W. (2015). Effect of citral on the cytotoxicity of doxorubicin in human B-lymphoma cells. Pharmaceutical Biology, 53, 262–268.
Fucikova, J., Kralikova, P., Fialova, A., et al. (2011). Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Research, 71, 4821–4833.
Obeid, M., Tesniere, A., Ghiringhelli, F., et al. (2007). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Medicine, 13, 54–61.
Sukkurwala, A. Q., Martins, I., Wang, Y., et al. (2014). Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell Death and Differentiation, 21, 59–68.
Zappasodi, R., Pupa, S. M., Ghedini, G. C., et al. (2010). Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Research, 70, 9062–9072.
Chao, M. P., Jaiswal, S., Weissman-Tsukamoto, R., et al. (2010). Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Science Translational Medicine, 2, 63–94.
Fucikova, J., Moserova, I., Truxova, I., et al. (2014). High hydrostatic pressure induces immunogenic cell death in human tumor cells. International Journal of Cancer, 135, 1165–1177.
Spisek, R., Charalambous, A., Mazumder, A., et al. (2007). Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood, 109, 4839–4845.
Spisek, R., & Dhodapkar, M. V. (2007). Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle, 6, 1962–1965.
Ma, Y., Adjemian, S., Mattarollo, S. R., et al. (2013). Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity, 38, 729–741.
Garg, A. D., Martin, S., Golab, J., et al. (2014). Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death and Differentiation, 21, 26–38.
Ayna, G., Krysko, D. V., Kaczmarek, A., et al. (2012). ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PloS One, 7, e40069.
Zitvogel, L., Kepp, O., Galluzzi, L., et al. (2012). Inflammasomes in carcinogenesis and anticancer immune responses. Nature Immunology, 13, 343–351.
Ghiringhelli, F., Apetoh, L., Tesniere, A., et al. (2009). Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature Medicine, 15, 1170–1178.
Michaud, M., Martins, I., Sukkurwala, A. Q., et al. (2011). Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science, 334, 1573–1577.
Ko, A., Kanehisa, A., Martins, I., et al. (2014). Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death and Differentiation, 21, 92–99.
Mandapathil, M., Hilldorfer, B., Szczepanski, M. J., et al. (2010). Generation and accumulation of immunosuppressive adenosine by human CD4 + CD25highFOXP3 + regulatory T cells. The Journal of Biological Chemistry, 285, 7176–7186.
Apetoh, L., Ghiringhelli, F., Tesniere, A., et al. (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Medicine, 13, 1050–1059.
Bell, C. W., Jiang, W., Reich, C. F., et al. (2006). The extracellular release of HMGB1 during apoptotic cell death. American Journal of Physiology Cell Physiology, 291, C1318-1325.
Morva, A., Lemoine, S., Achour, A., et al. (2012). Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood, 119(1), 106–114.
Lechmann, M., Berchtold, S., Hauber, J., & Steinkasserer, A. (2002). CD83 on dendritic cells: more than just a marker for maturation. TRENDS in Immunol, 23(6), 273–275.
Kawamura, K., Iyonaga, K., Ichiyasu, H., et al. (2005). Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clinical and Diagnostic Laboratory Immunology, 12, 206–212.
Casey, S. C., Tong, L., Li, Y., et al. (2016). MYC regulates the antitumor immune response through CD47 and PD-L1. Science, 352, 227–231.
Miyoshi, H., Kiyasu, J., Kato, T., et al. (2016). PD-L1 expression on neoplastic or stromal cell is respectively poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood, 128, 1374–1381.
Willingham, S. B., Volkmer, J. P., Gentles, A. J., et al. (2012). The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proceedings of the National Academy of Sciences of the United States of America, 109:6662–6667.
Fong, C. Y., Tam, K., Cheyyatraivendran, S., et al. (2014). Human Wharton’s jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. Journal of Cellular Biochemistry, 115, 290–302.
Paladini, L., Fabris, L., Bottai, G., et al. (2016). Targeting microRNAs as key modulators of tumor immune response. Journal of Experimental & Clinical Cancer Research, 35, 103.
Mehta, A., & Baltimore, D. (2016). MicroRNAs as regulatory elements in immune system logic. Nature Reviews Immunology, 16, 279–294.
Mei, J., Bachoo, R., & Zhang, C., L. (2011). MicroRNA-146a inhibits glioma development by targeting Notch1. Molecular Cell Biology, 31, 3584–3592.
Xu., B., Wang., N., Wang., X., et al. (2012). MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. The Prostate, 72, 1171–1178.
Chen, G., Umelo, I. A., Teugels, S.Lv., et al. (2013). miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One, 8(3), e60317.
Yu, Q., Liu, S. L., Wang, H., et al. (2013). miR-126 Suppresses the proliferation of cervical cancer cells and alters cell sensitivity to the chemotherapeutic drug bleomycin. Asian Pacific Journal Cancer Prevention, 14:11, 6569–6572.
Miko, E., Margitai, Z., Czimmerer, Z., et al. (2011). miR-126 inhibits proliferation of small cell lung cancer cells by targeting SLC7A5. FEBS Letters, 585(8), 1191–1196.
Du, C., Lv, Z., Cao, L., et al. (2014). MiR-126-3p suppresses tumor metastasis and angiogenesis of hepatocellular carcinoma by targeting LRP6 and PIK3R2. Journal of Transational Medicine, 12, 259.
Li, Z., Li, N., Wu, M., et al. (2013). Expression of miR-126 suppresses migration and invasion of colon cancer cells by targeting CXCR4. Molecular and Cell Biochemistry, 381(1–2), 233–243.
Acknowledgements
This work was supported by the Singapore National University Health System (NUHS) Aspiration Fund (New Idea) (R-174-000-155-720) research grant.
Author information
Authors and Affiliations
Contributions
DHL: Conception and design, executed the experiments, collection and assembly of data, analysis and interpretation, manuscript writing. ABi: Sought written informed consent from patients, obtained IRB approval for study, collection of human umbilical cords. MC: Data analysis and interpretation, scientific input. CYF: Conception and design, analysis and interpretation, scientific input, helped to obtain financial support. ABo: Conception and design, analysis and interpretation, manuscript correction and final approval, obtained financial support, Principal Investigator of the grant.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lin, D.H., Biswas, A., Choolani, M. et al. Induction of Immunogenic Cell Death in Lymphoma Cells by Wharton’s Jelly Mesenchymal Stem Cell Conditioned Medium. Stem Cell Rev and Rep 13, 801–816 (2017). https://doi.org/10.1007/s12015-017-9767-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9767-8