Abstract
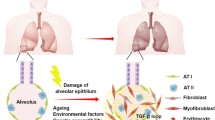
Idiopathic pulmonary fibrosis is a progressive fibrosing disorder for which there is no cure and no pharmacological treatment capable of increasing in a meaningful way the survival rate. Lung transplantation remains the only possible treatment for patients with advanced disease, although the increase in 5-year survival is only 45 %. Some preclinical studies have generated promising results about the therapeutic potential of exogenous stem cells. However, two initial clinical trials involving the endobronchial or systemic delivery of autologous adipose tissue-derived or unrelated-donor, placenta-derived mesenchymal stem cells have not convincingly demonstrated that these treatments are acceptably safe. The results of other ongoing clinical trials may help to identify the best source and delivery route of mesenchymal stem cells and to estimate the risk of unwanted effects related to the mesenchymal nature of the transplanted cells. Considering that most of the therapeutic potential of these cells has been ascribed to paracrine signaling, the use of mesenchymal stem cell-derived secretome as an alternative to the transplantation of single cell suspension may circumvent many regulatory and clinical problems. Technical and safety concerns still limit the possibility of clinical applications of other promising interventions that are based on the use of human amnion stem cells, embryonic stem cells or induced pluripotent stem cells to replace or regenerate the dysfunctional alveolar epithelium. We summarize the current status of the field and identify major challenges and opportunities for the possible future integration of stem cell-based treatments into the currently recommended clinical management strategy for idiopathic pulmonary fibrosis.



Similar content being viewed by others
References
Khalil, N., Churg, A., Muller, N., & O’Connor, R. (2007). Environmental, inhaled and ingested causes of pulmonary fibrosis. Toxicologic Pathology, 35, 86–96.
Wilson, M. S., & Wynn, T. A. (2009). Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunology, 2, 103–121.
Xu, J.-F., Washko, G. R., Nakahira, K., et al. (2012). Statins and pulmonary fibrosis. The potential role of NLRP3 inflammasome activation. American Journal of Respiratory and Critical Care Medicine, 185, 547–556.
Raghu, G., Collard, H. R., Egan, J. J., et al. (2011). An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine, 183, 788–824.
Nalysnyk, L., Cid-Ruzafa, J., Rotella, P., & Esser, D. (2012). Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. European Respiratory Review, 21, 355–361.
Ley, B., Collard, H. R., & King, T. E., Jr. (2011). Clinical course and prediction of survival in idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 183, 431–440.
Hunninghake, G. W., & Schwarz, M. I. (2007). Does current knowledge explain the pathogenesis of idiopathic pulmonary fibrosis? A perspective. Proceedings of the American Thoracic Society, 4, 449–452.
Moeller, A., Ask, K., Warburton, D., Gauldie, J., & Kolb, M. (2008). The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? The International Journal of Biochemistry & Cell Biology, 40, 362–382.
Moore, B. B., & Hogaboam, C. M. (2008). Murine models of pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology, 294, L152–L160.
Scotton, C. J., & Chamners, R. C. (2010). Bleomycin revisited: towards a more representative model of IPF? American Journal of Physiology. Lung Cellular and Molecular Physiology, 299, L439–L441.
Degryse, A. L., Tanjore, H., Xu, X. C., Polosukhin, V. V., Jones, B. R., McMahon, F. B., Gleaves, L. A., Blackwell, T. S., & Lawson, W. E. (2010). Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology, 299, L442–L452.
Degryse, A. L., & Lawson, W. E. (2011). Progress toward improving animal models for IPF. The American Journal of the Medical Sciences, 341, 444–449.
Raghu, G., Anstrom, K. J., King, T. E., Jr., Lasky, J. A., & Martinez, F. J. (2012). Prednisone, azathioprine, and N-acetylcisteine for pulmonary fibrosis. The New England Journal of Medicine, 366, 1968–1977.
Papiris, S. A., Manali, E. D., Kolilekas, L., et al. (2010). Clinical review: idiopathic pulmonary fibrosis acute exacerbations – unravelling Ariadne’s thread. Critical Care, 14, 246.
Konishi, K., Gibson, K. F., Lindell, K. O., et al. (2009). Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 180, 167–175.
Wootton, S. C., Kim, D. S., Kondoh, Y., et al. (2011). Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 183, 1698–1702.
Friedman, S. L., Sheppard, D., Duffield, J. S., & Violette, S. (2013). Therapy for fibrotic diseases: nearing the starting line. Science Translational Medicine, 5, 167sr1.
Hunninghake, G. M. (2014). A new hope for idiopathic pulmonary fibrosis. The New England Journal of Medicine, 370, 2142–2143.
Shorr, A.F. (2014). Exciting IPF treatment news must be interpreted cautiously. Medscape, http://www.medscape.com/viewarticle/828543. Accessed 24 July 2014.
Raghu, G., & Selman, M. (2015). Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. American Journal of Respiratory and Critical Care Medicine, 191, 252–254.
George, T. J., Arnaoutakis, G. J., & Shah, A. S. (2011). Lung transplant in idiopathic pulmonary fibrosis. Archives of Surgery, 146, 1204–1209.
Weiss, D. J. (2013). Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010–2012. Annals of the American Thoracic Society, 10, S45–S97.
Toonkel, R. L., Hare, J. M., Matthay, M. A., & Glassberg, M. K. (2013). Mesenchymal stem cells and idiopathic pulmonary fibrosis. Potential for clinical testing. American Journal of Respiratory and Critical Care Medicine, 188, 133–140.
Weiss, D. J., & Ortiz, L. A. (2013). Cell therapy trials for lung diseases: progress and cautions. American Journal of Respiratory and Critical Care Medicine, 188, 123–125.
McNulty, K., & Janes, S. M. (2012). Stem cells and pulmonary fibrosis: cause or cure? Proceedings of the American Thoracic Society, 9, 164–171.
Wetsel, R. A., Wang, D., & Calame, D. G. (2011). Therapeutic potential of lung epithelial progenitor cells derived from embryonic and induced pluripotent stem cells. Annual Review of Medicine, 62, 95–105.
Lutolf, M. P., Gilbert, P. M., & Blau, H. M. (2009). Designing materials to direct stem-cell fate. Nature, 462, 433–441.
American Thoracic Society, & European Respiratory Society. (2002). American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. American Journal of Respiratory and Critical Care Medicine, 165, 277–304.
Visscher, D. W., & Myers, J. L. (2006). Histologic spectrum of idiopathic interstitital pneumonias. Proceedings of the American Thoracic Society, 3, 322–329.
Barbas-Filho, J. V., Ferreira, M. A., Sesso, A., Kairalla, R. A., Carvalho, C. R. R., & Capelozzi, V. L. (2001). Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP). Journal of Clinical Pathology, 54, 132–138.
Waisberg, D. R., Barbas-Filho, J. V., Parra, E. R., et al. (2010). Abnormal expression of telomerase/apoptosis limits type II alveolar epithelial cell replication in the early remodeling of usual interstitial pneumonia/idiopathic pulmonary fibrosis. Human Pathology, 41, 385–391.
Selman, M., & Pardo, A. (2006). Role of epithelial cells in idiopathic pulmonary fibrosis. From innocent targets to serial killers. Proceedings of the American Thoracic Society, 3, 364–372.
Katzenstein, A.-L., & Myers, J. L. (1998). Idiopathic pulmonary fibrosis. Clinical relevance of pathologic classification. State of the art. American Journal of Respiratory and Critical Care Medicine, 157, 1301–1315.
Seibold, M. A., Smith, R. W., Urbanek, C., et al. (2013). The idiopathic pulmonary fibrosis honeycomb cyst contains a mucociliary pseudostratified epithelium. PLoS One, 8, e58658.
Camelo, A., Dunmore, R., Sleeman, M. S., & Clarke, D. L. (2014). The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Frontiers in Pharmacology, 4, 173.
Sakai, N., & Tager, A. M. (2013). Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochimica et Biophysica Acta, 1832, 911–921.
Garcia, C. K. (2011). Idiopathic pulmonary fibrosis: update on genetic discoveries. Proceedings of the American Thoracic Society, 8, 158–162.
Kropski, J. A., Lawson, W. E., Young, L. R., & Blackwell, T. S. (2013). Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Disease Models & Mechanics, 6, 9–17.
Steele, M. P., & Schwartz, D. A. (2013). Molecular mechanisms in progressive idiopathic pulmonary fibrosis. Annual Review of Medicine, 64, 265–276.
Armanios, M. (2013). Telomeres and age-related disease: how telomere biology informs clinical paradigms. The Journal of Clinical Investigation, 123, 996–1002.
Fingerlin, T. E., Murphy, E., Zhang, W., et al. (2013). Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nature Genetics, 45, 613–620.
Eickelberg, O., & Hunninghake, G. M. (2015). A first glimpse at the early origins of idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 191, 366–368.
Adamson, Y. R., Young, L., & Bowden, D. H. (1988). Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. The American Journal of Pathology, 130, 377–383.
Coxson, H. O., Hogg, J. C., Mayo, J. R., et al. (1997). Quantification of idiopathic pulmonary fibrosis using computed tomography and histology. American Journal of Respiratory and Critical Care Medicine, 155, 1649–1656.
Liu, T., Warburton, R. R., Guevara, O. E., et al. (2007). Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 37, 507–517.
Takeji, M., Moriyama, T., Oseto, S., et al. (2006). Smooth muscle α-actin deficiency in myofibroblasts leads to enhanced renal fibrosis. The Journal of Biological Chemistry, 281, 40193–40200.
Cieslik, K. A., Trial, J., Carlson, S., Taffet, G. E., & Entman, M. L. (2013). Aberrant differentiation of fibroblast progenitors contribute to fibrosis in the aged murine heart: role of elevated circulating insulin levels. The FASEB Journal, 27, 1761–1771.
Bianchetti, L., Barczyk, M., Cardoso, J., Schmidt, M., Bellini, A., & Mattoli, S. (2012). Extracellular matrix remodelling properties of human fibrocytes. Journal of Cellular and Molecular Medicine, 16, 483–495.
Mattoli, S., Bellini, A., & Schmidt, M. (2009). The role of a human hematopoietic mesenchymal progenitor in wound healing and fibrotic diseases and implications for therapy. Current Stem Cell Research & Therapy, 4, 266–280.
Bellini, A., & Mattoli, S. (2010). Fibrocytes (reactive or reparative). In J. Polak (Ed.), Cell therapy for lung disease (pp. 237–252). London: Imperial College Press.
Keeley, E. C., Mehrad, B., & Strieter, R. M. (2011). The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis & Tissue Repair, 4, 2.
Ellson, C. D., Dunmore, R., Hogaboam, C. M., Sleeman, M. A., & Murray, L. A. (2014). Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 51, 163–168.
Bianchetti, L., Marini, M. A., Isgrò, M., Bellini, A., Schmidt, M., & Mattoli, S. (2012). IL-33 promotes the migration and proliferation of circulating fibrocytes from patients with allergen-exacerbated asthma. Biochemical and Biophysical Research Communications, 426, 116–121.
Xia, H., Bodempudi, V., Benyumov, A., et al. (2014). Identification of a cell-of-origin for fibroblasts comprising the fibrotic reticulum in idiopathic pulmonary fibrosis. The American Journal of Pathology, 184, 1369–1383.
Lau, A. N., Goodwin, M., Kim, C. F., & Weiss, D. J. (2012). Stem cells and regenerative medicine in lung biology and diseases. Molecular Therapy, 20, 1116–1130.
Serrano-Mollar, A., Nacher, M., Gay-Jordi, G., Closa, D., Xaubet, A., & Bulbena, O. (2007). Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. American Journal of Respiratory and Critical Care Medicine, 176, 1261–1268.
Garcia, O., Carraro, G., Navarro, S., et al. (2012). Cell-based therapies for lung disease. British Medical Bulletin, 101, 147–161.
Murphy, M. B., Moncivais, K., & Caplan, A. I. (2013). Mesenchymal stem cells: environmentally responsive therapeutic for regenerative medicine. Experimental & Molecular Medicine, 45, e54.
Baer, P. C. (2014). Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World Journal of Stem Cells, 6, 256–265.
Caruso, M., Evangelista, M., & Parolini, O. (2012). Human term placental cells: phenotype, properties and new avenues in regenerative medicine. International Journal of Molecular and Cellular Medicine, 1, 64–74.
Cyranoski, D. (2012). Canada approves stem cell product. Nature Biotechnology, 30, 571.
Lalu, M. M., McIntyre, L., Pugliese, C., et al. (2012). Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One, 7, e47559.
von Bahr, L., Batsis, I., Moll, G., et al. (2012). Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells, 30, 1575–1578.
Yang, J., & Jia, Z. (2014). Cell-based therapy in lung regenerative medicine. Regenerative Medicine Research, 2, 7.
Islam, M. N., Das, S. R., Emin, M. T., et al. (2012). Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Medicine, 18, 759–765.
Ortiz, L. A., Gambelli, F., McBride, C., et al. (2003). Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceeding of the National Academy of Sciences of the United States of America, 100, 8407–8411.
Rojas, M., Xu, J., Woods, C. R., et al. (2005). Bone marrow-derived mesenchymal stem cells in repair of the injured lung. American Journal of Respiratory Cell and Molecular Biology, 33, 145–152.
Zhao, F., Zhang, Y. F., Liu, Y. G., et al. (2008). Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplantation Proceedings, 40, 1700–1705.
Kumamoto, M., Nishiwaki, T., Matsuo, N., Kimura, H., & Matsushima, K. (2009). Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. European Respiratory Journal, 34, 740–748.
Lee, S. H., Jang, A. S., Kim, Y. E., et al. (2010). Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respiratory Research, 11, 16.
Aguilar, S., Scotton, C. J., McNulty, K., et al. (2009). Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin induced pulmonary fibrosis. PLoS One, 4, e8013.
Moodley, Y., Atienza, D., Manuelpillai, U., et al. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis in bleomycin-induced lung injury. American Journal of Pathology, 175, 303–313.
Saito, S., Nakayama, T., Hashimoto, N., et al. (2011). Mesenchymal stem cells stably transduced with a dominant-negative inhibitor of CCL2 greatly attenuated bleomycin-induced lung damage. American Journal of Pathology, 179, 1088–1094.
Lim, R., Miltonic, P., Murphy, S., Dickinson, H., Chan, S. T., & Jenkin, G. (2013). Human mesenchymal stem cells reduce lung injury in immunocompromised mice but not in immunocompetent mice. Respiration, 85, 332–341.
Gazdhar, A., Susuri, N., Hostettler, K., et al. (2013). HGF expressing stem cells in usual interstitial pneumonia originate from the bone marrow and are antifibrotic. PLoS One, 8, e65453.
Lee, S. H., Lee, E. J., Lee, S. Y., et al. (2014). The effect of adipose stem cell therapy on pulmonary fibrosis induced by repetitive intratracheal bleomycin in mice. Experimental Lung Research, 40, 117–125.
Moodley, Y., Ilancheran, S., Samuel, C., et al. (2010). Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. American Journal of Respiratory and Critical Care Medicine, 182, 643–651.
Murphy, S., Lim, R., Dickinson, H., et al. (2011). Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplantation, 20, 909–923.
Cargnoni, A., Gibelli, L., Tosini, A., et al. (2009). Transplantation of allogenic and xenogenic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplantation, 18, 405–422.
Wang, D., Morales, J. E., Calame, D. G., Alcom, J. L., & Wetsel, R. A. (2010). Transplantation of human embryonic stem cell-derived alveolar epithelial type II epithelial cells abrogates acute lung injury in mice. Molecular Therapy, 18, 625–634.
Soh, B. S., Zheng, D., Yeo, J. S. L., et al. (2012). CD166(pos) subpopulation from differentiated human ES and iPS cells support repair of acute lung injury. Molecular Therapy, 20, 2335–2346.
Bauer, Y., Tedrow, J., de Bernard, S., et al. (2015). A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 52, 217–231.
Ortiz, L. A., Dutreil, M., Fattman, C., et al. (2007). Interleukin 1 receptor antagonist mediates the anti-inflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceeding of the National Academy of Sciences of the United States of America, 104, 11002–11007.
Yan, X., Liu, Y., Han, Q., et al. (2007). Injured microenvironment directly guides the differentiation of engrafted flk-1(+) mesenchymal stem cell in lung. Experimental Hematology, 35, 1466–1475.
Epperly, M. W., Guo, H., Gretton, J. E., & Greenberger, J. S. (2003). Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 29, 213–224.
Tzouvelekis, A., Paspaliaris, V., Koliakos, G., et al. (2013). A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Journal of Translational Medicine, 11, 171.
Chambers, D. C., Enever, D., Ilic, N., et al. (2014). A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology, 19, 1013–1018.
Bourin, P., Bunnell, B. A., Casteilla, L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15, 641–648.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Yuan, B. Z., & Wang, J. (2014). The regulatory sciences for stem cell-based medicinal products. Frontiers of Medicine, 8, 190–200.
Brooke, G., Rossetti, T., Pelekanos, R., et al. (2009). Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. British Journal of Haematology, 144, 571–579.
Ilic, N., Brooke, G., Murray, P., et al. (2011). Manufacture of clinical grade human placenta-derived multipotent mesenchymal stromal cells. Methods in Molecular Biology, 698, 89–106.
De Coppi, P., Bartsch, G., Jr., Siddiqui, M. M., et al. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology, 25, 100–106.
Perin, L., Giuliani, S., Jin, D., et al. (2007). Renal differentiation of amniotic fluid stem cells. Cell Proliferation, 40, 936–948.
Carraro, G., Perin, L., Sedrakyan, S., et al. (2008). Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells, 26, 2902–2911.
Mirabella, T., Poggi, A., Scaranari, M., et al. (2011). Recruitment of host’s progenitor cells to sites of human amniotic fluid stem cells implantation. Biomaterials, 32, 4218–4227.
Angelini, A., Castellani, C., Ravara, B., et al. (2011). Stem-cell therapy in an experimental model of pulmonary hypertension and right heart failure: role of paracrine and neurohormonal milieu in the remodelling process. The Journal of Heart and Lung Transplantation, 30, 1281–1293.
Ilancheran, S., Michalska, A., Peh, G., Wallace, E. M., Pera, M., & Manuelpillai, U. (2007). Stem cells derived from human fetal membranes display multi-lineage differentiation potential. Biology of Reproduction, 77, 577–588.
Da Sacco, S., Sedrakyan, S., Boldrin, F., et al. (2010). Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. The Journal of Urology, 183, 1193–1200.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147.
Wang, D., Haviland, D. L., Burns, A. R., Zsigmond, E., & Wetsel, R. A. (2007). A pure population of lung alveolar epithelial II cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104, 4449–4454.
Spitalieri, P., Quitadamo, M. C., Orlandi, A., et al. (2012). Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. European Respiratory Journal, 39, 446–457.
Roszell, B., Mondrinos, M. J., Seaton, A., et al. (2009). Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Engineering Part A, 15, 3351–3365.
Longmire, T. A., Ikonomou, L., Hawkins, F., et al. (2012). Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell, 10, 398–411.
Lin, Y. M., Zhang, A., Rippon, H. J., Bismarck, A., & Bishop, A. E. (2010). Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Engineering Part A, 16, 1515–1526.
Wang, Y., Wong, L. B., & Mao, H. (2010). Induction of ciliated cells from avian embryonic stem cells using three-dimensional matrix. Tissue Engineering. Part C, Methods, 16, 929–936.
Baker, D. E., Harrison, N. J., Maltby, E., et al. (2007). Adaptation to culture of human embryonic stem cells and oncogenenesis in vivo. Nature Biotechnology, 25, 207–215.
Fehrenbach, F. (2012). Alveolar epithelial type II cells from embryonic stem cells: knights in shining armour? European Respiratory Journal, 39, 240–241.
Takahashi, K., Tanabe, K., Ohnuki, M., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920.
Nishikawa, S., Goldstein, R. A., & Nierras, C. R. (2008). The promise of human induced pluripotent stem cells for research and therapy. Nature Reviews Molecular Cell Biology, 9, 725–729.
Green, M. D., Chen, A., Nostro, M. C., et al. (2011). Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature Biotechnology, 29, 267–272.
Mou, H., Zhao, R., Sherwood, R., et al. (2012). Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell, 10, 385–397.
Ghaedi, M., Calle, E. A., Mendez, J. J., et al. (2013). Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. The Journal of Clinical Investigation, 123, 4950–4962.
Kiskinis, E., & Eggan, K. (2010). Progress toward the clinical application of patient-specific plutipotent stem cells. The Journal of Clinical Investigation, 120, 51–59.
Pei, D., Xu, J., Zhuang, Q., Tse, H. F., & Esteban, M. A. (2010). Induced pluripotent stem cell technology in regenerative medicine and biology. Advances in Biochemical Engineering/Biotechnology, 123, 127–141.
Barrilleaux, B., & Knoepfler, P. S. (2011). Inducing IPSCs to escape the dish. Cell Stem Cell, 9, 103–111.
Zhao, T., Zhang, Z.-N., Rong, Z., & Xu, Y. (2011). Immunogenicity of induced pluripotent stem cells. Nature, 474, 212–215.
Soldner, F., Hockemeyer, D., Beard, C., et al. (2009). Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell, 136, 964–977.
Kaji, K., Norrby, K., Paca, A., Mileikovsky, M., Mohseni, P., & Woltjen, K. (2009). Virus free induction of pluripotency and subsequent excision of reprogramming factors. Nature, 458, 771–775.
Woltjen, K., Michael, I. P., Mohseni, P., et al. (2009). piggiBac transposition reprograms fibroblasts to induced plutipotent stem cells. Nature, 458, 766–770.
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G., & Hoch-Edlinger, K. (2008). Induced pluripotent stem cells generated without viral integration. Science, 322, 945–949.
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T., & Yamanaka, S. (2008). Generation of mouse induced pluripotent stem cells without viral vectors. Science, 322, 949–953.
Zhou, H., Wu, S., Joo, J. Y., et al. (2009). Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell, 4, 381–384.
Gonzalez, F., Barragan Monasterio, M., Tiscornia, G., et al. (2009). Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proceedings of the National Academy of Sciences of the United States of America, 106, 8918–8922.
Kim, D., Kim, C. H., Moon, J. I., et al. (2009). Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell, 4, 472–476.
Yu, J., Hu, K., Smuga-Otto, K., et al. (2009). Human induced pluripotent stem cells free of vector and transgene sequences. Science, 324, 797–801.
European Medicines Agency, Committee for Advanced Therapies. (2010). Reflection paper on stem cell-based medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500079932.pdf. Accessed 23 September 2014.
George, B. (2011). Regulations and guidelines governing stem cell based products: clinical considerations. Perspectives in Clinical Research, 2, 94–99.
Viswanathan, S., Keating, A., Deans, R., et al. (2014). Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells and Development, 23, 1157–1167.
Collins, E., Gu, F., Qi, M., et al. (2014). Differential efficacy of human mesenchymal stem cells based on source of origin. The Journal of Immunology, 193, 4381–4390.
Schinköthe, T., Bloch, W., & Schmidt, A. (2008). In vitro secreting profile of human mesenchymal stem cells. Stem Cells and Development, 17, 199–206.
Lai, R. C., Chen, T. S., & Lim, S. K. (2011). Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative Medicine, 6, 481–492.
Ranganath, S. H., Levy, O., Inamdar, M. S., & Karp, J. M. (2012). Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell, 10, 244–258.
Ionescu, L., Byrne, R. N., van Haaften, T., et al. (2012). Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. American Journal of Physiology. Lung Cellular and Molecular Physiology, 303, L967–L977.
Lai, R. C., Tan, S. S., Teh, B. J., et al. (2012). Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. International Journal of Proteomics, 2012, 971907.
Kim, H. S., Choi, D. Y., Yun, S. J., et al. (2012). Proteomic analysis of microvesicles derived from human mesenchymal stem cells. Journal of Proteome Research, 11, 839–849.
Lee, C., Mitsialis, S. A., Aslam, M., et al. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 126, 2601–2611.
Li, T., Yan, Y., Wang, B., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells and Development, 22, 845–854.
Zhou, Y., Xu, H., Xu, W., et al. (2013). Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Research Therapy, 4, 34.
Arslan, F., Lai, R. C., Smeets, M. B., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 10, 301–312.
Akyurekli, C., Le, Y., Richardson, R. B., Fergusson, D., Tay, J., & Allan, D. S. (2014). A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Reviews and Reports. doi:10.1007/s12015-014-9545-9.
Lai, R. C., Arslan, F., Tan, S. S., et al. (2010). Derivation and characterization of human fetal MSCs: an alternative source for large-scale production of cardioprotective microparticles. Journal of Molecular and Cellular Cardiology, 48, 1215–1224.
Lai, R. C., Yeo, R. W. Y., Tan, K. H., & Lim, S. K. (2013). Exosomes for drug delivery – a novel application for the mesenchymal stem cell. Biotechnology Advances, 31, 543–551.
Ly, H. (2011). Telomere dynamics in induced pluripotent stem cells: potentials for human disease modeling. World Journal of Stem Cells, 3, 89–95.
Mooney, D. J., & Vandenburgh, H. (2008). Cell delivery mechanisms for tissue repair. Cell Stem Cell, 2, 205–213.
Discher, D. E., Mooney, D. J., & Zandstra, P. W. (2009). Growth factors, matrices, and forces combine and control stem cells. Science, 324, 1673–1677.
Wagner, D. E., Bonenfant, N. R., Parsons, C. S., et al. (2014). Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials, 35, 3281–3297.
Booth, A. J., Hadley, R., Cornett, A. M., et al. (2012). Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. American Journal of Respiratory and Critical Care Medicine, 186, 866–876.
Williams, K., Malarkey, D., Cohn, L., Patrick, D., Dye, J., & Toews, G. (2004). Identification of spontaneous feline idiopathic pulmonary fibrosis. Morphology, and ultrastructural evidence for a type II pneumocyte defect. Chest, 125, 2278–2288.
Roman, J., Brown, K. K., Olson, A., Corcoran, B. M., Williams, K. J., & on behalf of the ATS Comparative Biology of Lung Fibrosis Working Group. (2013). An official American Thoracic Society workshop report: Comparative pathobiology of fibrosing lung disorders in humans and domestic animals. Annals of the American Thoracic Society, 10, S224–S229.
Chaudhary, N. I., Schnapp, A., & Park, J. E. (2006). Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. American Journal of Respiratory and Critical Care Medicine, 173, 769–776.
Schaefer, C. J., Ruhrmund, D. W., Pan, L., Seiwert, S. D., & Kossen, K. (2011). Antifibrotic activities of pirfenidone in animal models. European Respiratory Review, 20, 85–97.
Churg, A., Wright, J. L., & Tazelaar, H. D. (2011). Acute exacerbations of fibrotic interstitial lung disease. Histopathology, 58, 525–530.
Song, J. J., & Ott, H. C. (2012). Bioartificial lung engineering. American Journal of Transplantation, 12, 283–288.
Gutierrez-Soto, A., Wertheim, J. A., Ott, H. C., & Gilbert, T. W. (2012). Perspectives on whole-organ assembly: moving toward transplantation on demand. The Journal of Clinical Investigation, 122, 3817–3823.
De Miquel, M. P., Fuentes-Julián, S., Blázquez-Martínez, A., et al. (2012). Immunosuppressive properties of mesenchymal stem cells: advances and applications. Current Molecular Medicine, 12, 574–591.
Wang, J., Liao, L., & Tan, J. (2011). Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opinion Biological Therapeutics, 11, 893–909.
Silhan, L. L., Shah, P. D., Chambers, D. C., et al. (2014). Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. European Respiratory Journal, 44, 178–187.
Conflict of Interest
SM is founding shareholder and board member of AVAIL GmbH. The other authors have no conflicts of interest to disclose in addition to their affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barczyk, M., Schmidt, M. & Mattoli, S. Stem Cell-Based Therapy in Idiopathic Pulmonary Fibrosis. Stem Cell Rev and Rep 11, 598–620 (2015). https://doi.org/10.1007/s12015-015-9587-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9587-7