Abstract
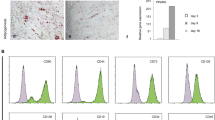
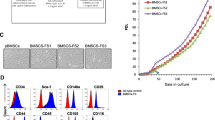
Mouse embryonic fibroblasts have been utilized as a surrogate stem cell model for the postnatal bone marrow-derived stromal stem cells (BMSC) to study mesoderm-type cell differentiation e.g. osteoblasts, adipocytes and chondrocytes. However, no formal characterization of MEF phenotype has been reported. Utilizing standard in vitro and in vivo assays we performed a side-by-side comparison of MEF and BMSC to determine their ability to differentiate into mesoderm-type cells. BMSC were isolated from 8–10 weeks old mouse bone marrow by plastic adherence. MEF were established by trypsin/EDTA digestion from E13.5 embryos after removing heads and viscera, followed by plastic adherence. Compared to BMSC, MEF exhibited telomerase activity and improved cell proliferation as assessed by q-PCR based TRAP assay and cell number quantification, respectively. FACS analysis revealed that MEF exhibited surface markers characteristic of the BMSC: Sca-1+, CD73+, CD105+, CD29+, CD44+, CD106+, CD11b−, and CD45−. In contrast to BMSC, ex vivo osteoblast (OB) differentiation of MEF exhibited a less mature osteoblastic phenotype (less alkaline phosphatase, collagen type I and osteocalcin) as assessed by real-time PCR analysis. Compared to BMSC, MEF exhibited a more enhanced differentiation into adipocyte and chondrocyte lineages. Interestingly, both MEF and BMSC formed the same amount of heterotopic bone and bone marrow elements upon in vivo subcutaneous implantation with hydroxyapatite/tricalcium phosphate, in immune deficient mice. In conclusion, MEF contain a population of stem cells that behave in ex vivo and in vivo assays, similar but not identical, to BMSC. Due to their enhanced cell growth, they may represent a good alternative for BMSC in studying molecular mechanisms of stem cell commitment and differentiation to osteoblasts, adipocytes and chondrocytes.





Similar content being viewed by others
References
Bianco, P., Kuznetsov, S. A., Riminucci, M., & Gehron, R. P. (2006). Postnatal skeletal stem cells. Methods in Enzymology, 419, 117–148.
Kassem, M. (2004). Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning and Stem Cells, 6(4), 369–374.
Kuroda, Y., Kitada, M., Wakao, S., et al. (2010). Unique multipotent cells in adult human mesenchymal cell populations. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8639–8643.
Post, S., Abdallah, B. M., Bentzon, J. F., & Kassem, M. (2008). Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone, 43(1), 32–39.
Zhou, S. (2011). TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Saeed, H., Abdallah, B. M., Ditzel, N., et al. (2011). Telomerase-deficient mice exhibit bone loss due to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. Journal of Bone and Mineral Research.
Simonsen, J. L., Rosada, C., Serakinci, N., et al. (2002). Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nature Biotechnology, 20(6), 592–596.
Peister, A., Mellad, J. A., Larson, B. L., Hall, B. M., Gibson, L. F., & Prockop, D. J. (2004). Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood, 103(5), 1662–1668.
Phinney, D. G., Kopen, G., Isaacson, R. L., & Prockop, D. J. (1999). Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. Journal of Cellular Biochemistry, 72(4), 570–585.
Kuznetsov, S. A., Mankani, M. H., Gronthos, S., Satomura, K., Bianco, P., & Robey, P. G. (2001). Circulating skeletal stem cells. The Journal of Cell Biology, 153(5), 1133–1140.
Rossi, O., Barbieri, O., & Frosina, G. (2003). Time-course of spontaneous transformation of CD-1 mouse embryonic fibroblasts. Anticancer Research, 23(2B), 1373–1377.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Sun, H., Gulbagci, N. T., & Taneja, R. (2007). Analysis of growth properties and cell cycle regulation using mouse embryonic fibroblast cells. Methods in Molecular Biology (383), 311–319.
Ciruna, B. G., Schwartz, L., Harpal, K., Yamaguchi, T. P., & Rossant, J. (1997). Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development, 124(14), 2829–2841.
Deng, C. X., Wynshaw-Boris, A., Shen, M. M., Daugherty, C., Ornitz, D. M., & Leder, P. (1994). Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes & Development, 8(24), 3045–3057.
Kamijo, T., Zindy, F., Roussel, M. F., et al. (1997). Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell, 91(5), 649–659.
Lengner, C. J., Lepper, C., van Wijnen, A. J., Stein, J. L., Stein, G. S., & Lian, J. B. (2004). Primary mouse embryonic fibroblasts: a model of mesenchymal cartilage formation. Journal of Cellular Physiology, 200(3), 327–333.
Lowe, S. W., Jacks, T., Housman, D. E., & Ruley, H. E. (1994). Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proceedings of the National Academy of Sciences of the United States of America, 91(6), 2026–2030.
Steinman, H. A., Sluss, H. K., Sands, A. T., Pihan, G., & Jones, S. N. (2004). Absence of p21 partially rescues Mdm4 loss and uncovers an antiproliferative effect of Mdm4 on cell growth. Oncogene, 23(1), 303–306.
Alexander, D. L., Ganem, L. G., Fernandez-Salguero, P., Gonzalez, F., & Jefcoate, C. R. (1998). Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. Journal of Cell Science, 111(Pt 22), 3311–3322.
Yun, Z., Maecker, H. L., Johnson, R. S., & Giaccia, A. J. (2002). Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Developmental Cell, 2(3), 331–341.
Garreta, E., Genove, E., Borros, S., & Semino, C. E. (2006). Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Engineering, 12(8), 2215–2227.
Peister, A., Mellad, J. A., Larson, B. L., Hall, B. M., Gibson, L. F., & Prockop, D. J. (2004). Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood, 103(5), 1662–1668.
Post, S., Abdallah, B. M., Bentzon, J. F., & Kassem, M. (2008). Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone, 43(1), 32–39.
Zhang, Y., Zhao, L., Wang, C., & Lei, B. (2003). Isolation and culture of mouse embryonic fibroblast. Sichuan Da Xue Xue Bao. Yi Xue Ban, 34(2), 344–346.
Hogan, B., Beddington, R., Costantini, F., & Lacy, E. (1994). Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor Press.
Dimri, G. P., Lee, X., Basile, G., et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92(20), 9363–9367.
Johnstone, B., Hering, T. M., Caplan, A. I., Goldberg, V. M., & Yoo, J. U. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238(1), 265–272.
Abdallah, B. M., Ditzel, N., & Kassem, M. (2008). Assessment of bone formation capacity using in vivo transplantation assays: procedure and tissue analysis. Methods in Molecular Biology, 455, 89–100.
Abdallah, B. M., Haack-Sorensen, M., Burns, J. S., et al. (2005). Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochemical and Biophysical Research Communications, 326(3), 527–538.
Baddoo, M., Hill, K., Wilkinson, R., et al. (2003). Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. Journal of Cellular Biochemistry, 89(6), 1235–1249.
Foster, L. J., Zeemann, P. A., Li, C., Mann, M., Jensen, O. N., & Kassem, M. (2005). Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells, 23(9), 1367–1377.
Blasco, M. A., Lee, H. W., Hande, M. P., et al. (1997). Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell, 91(1), 25–34.
Itahana, K., Campisi, J., & Dimri, G. P. (2004). Mechanisms of cellular senescence in human and mouse cells. Biogerontology, 5(1), 1–10.
Stenderup, K., Justesen, J., Clausen, C., & Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919–926.
Campisi, J. (2011). Cellular senescence: putting the paradoxes in perspective. Current Opinion in Genetics & Development, 21(1), 107–112.
Wagner, W., Wein, F., Seckinger, A., et al. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology, 33(11), 1402–1416.
Larsen, K. H., Frederiksen, C. M., Burns, J. S., Abdallah, B. M., & Kassem, M. (2010). Identifying a molecular phenotype for bone marrow stromal cells with in vivo bone-forming capacity. Journal of Bone and Mineral Research, 25(4), 796–808.
Rutherford, R. B., Moalli, M., Franceschi, R. T., Wang, D., Gu, K., & Krebsbach, P. H. (2002). Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Engineering, 8(3), 441–452.
Wang, E. A., Israel, D. I., Kelly, S., & Luxenberg, D. P. (1993). Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors, 9(1), 57–71.
Abdallah, B. M., & Kassem, M. (2008). Human mesenchymal stem cells: from basic biology to clinical applications. Gene Therapy, 15(2), 109–116.
Bianco, P., Riminucci, M., Gronthos, S., & Robey, P. G. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells, 19(3), 180–192.
Barry, F., Boynton, R. E., Liu, B., & Murphy, J. M. (2001). Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Experimental Cell Research, 268(2), 189–200.
Goldring, M. B., Tsuchimochi, K., & Ijiri, K. (2006). The control of chondrogenesis. Journal of Cellular Biochemistry, 97(1), 33–44.
Lefebvre, V., & Smits, P. (2005). Transcriptional control of chondrocyte fate and differentiation. Birth Defects Research. Part C, Embryo Today, 75(3), 200–212.
Acknowledgements
The authors are grateful to Lone Christiansen for excellent technical assistance. The study was supported by grants from the Lundbeck foundation, the NovoNordisk Foundation, the Karen Elise Jensen’s foundation, Denmark and King Abdulaziz City for Science and Technology (09-BIO740-20), KSA. H. Saeed has received a PhD fellowship from the NovoNordisk Foundation, Denmark.
Conflict of interests
Authors have no conflicting interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.65 MB)
Rights and permissions
About this article
Cite this article
Saeed, H., Taipaleenmäki, H., Aldahmash, A.M. et al. Mouse Embryonic Fibroblasts (MEF) Exhibit a Similar but not Identical Phenotype to Bone Marrow Stromal Stem Cells (BMSC). Stem Cell Rev and Rep 8, 318–328 (2012). https://doi.org/10.1007/s12015-011-9315-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9315-x