Abstract
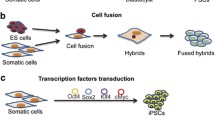
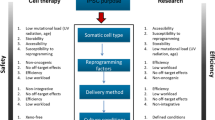
Pluripotent stem cells are basic cells with an indefinite self-renewal capacity and the potential to generate all the cell types of the three germinal layers. So far, the major source for pluripotent stem cells is the inner cell mass of the blastocysts: embryonic stem (ES) cells. Potential clinical application of ES cells is faced with many practical and ethical concerns. So, a major breakthrough was achieved in 2006, when it was shown that pluripotent stem cells could be obtained by transducing mouse embryonic and adult fibroblasts with a limited set of defined transcription factors. These reprogrammed cells, named induced pluripotent stem (iPS) cells, resembled ES cells in many of their characteristics. Since this initial study, iPS cell research has taken an incredible flight, and to date iPS cells have been generated from cells from several species using different sets of reprogramming factors. Given the potential to generate patient-specific cell populations without the need for human embryonic cells, iPS cell technology has been received with great excitement by research and medical communities. However, many questions regarding the actual molecular process of induced reprogramming remain unanswered and need to be addressed before iPS cells can go to the clinic. In this review, we start by summarizing recent advances in iPS cell research and inventory the hurdles that still need to be taken before safe clinical application. Our major aim, however, is to review the available data on the molecular processes underlying pluripotency reprogramming and present a two-stage switch model.





Similar content being viewed by others
References
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. doi:10.1016/j.cell.2006.07.024.
Tokuzawa, Y., Kaiho, E., Maruyama, M., et al. (2003). Fbx15 is a novel target of Oct3/4 but is dispensable for embryonic stem cell self-renewal and mouse development. Molecular and Cellular Biology, 23, 2699–2708. doi:10.1128/MCB.23.8.2699-2708.2003.
Nichols, J., Zevnik, B., Anastassiadis, K., et al. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell, 95, 379–391. doi:10.1016/S0092-8674(00)81769-9.
Mitsui, K., Tokuzawa, Y., Itoh, H., et al. (2003). The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell, 113, 631–642. doi:10.1016/S0092-8674(03)00393-3.
Maherali, N., Sridharan, R., Xie, W., et al. (2007). Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell, 1, 55–70. doi:10.1016/j.stem.2007.05.014.
Okita, K., Ichisaka, T., & Yamanaka, S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature, 448, 313–317. doi:10.1038/nature05934.
Wernig, M., Meissner, A., Foreman, R., et al. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 448, 318–324. doi:10.1038/nature05944.
Takahashi, K., Tanabe, K., Ohnuki, M., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872. doi:10.1016/j.cell.2007.11.019.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920. doi:10.1126/science.1151526.
Park, I. H., Zhao, R., West, J. A., et al. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451, 141–146. doi:10.1038/nature06534.
Mali, P., Ye, Z., Hommond, H. H., et al. (2008). Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells, 26, 1998–2005. doi:10.1634/stemcells.2008-0346.
Lowry, W. E., Richter, L., Yachechko, R., et al. (2008). Generation of human induced pluripotent stem cells from dermal fibroblasts. Proceedings of the National Academy of Sciences of the United States of America, 105, 2883–2888. doi:10.1073/pnas.0711983105.
Liao, J., Wu, Z., Wang, Y., et al. (2008). Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell Research, 18, 600–603. doi:10.1038/cr.2008.51.
Nakagawa, M., Koyanagi, M., Tanabe, K., et al. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology, 26, 101–106. doi:10.1038/nbt1374.
Dimos, J., Rodolfa, K., Niakan, K., et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science, 321, 1218–1221. doi:10.1126/science.1158799.
Park, I. H., Arora, N., Huo, H., et al. (2008). Disease-specific induced pluripotent stem cells. Cell, 134, 877–886. doi:10.1016/j.cell.2008.07.041.
Maherali, N., Ahfeldt, T., Rigamonti, A., Utikal, J., Cowan, C., & Hochedlinger, K. (2008). A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell, 3, 340–345. doi:10.1016/j.stem.2008.08.003.
Aasen, T., Raya, A., Barrero, M., et al. (2008). Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotechnology, 26, 1276–1284. doi:10.1038/nbt.1503.
Huangfu, D., Osafune, K., Maehr, R., et al. (2008). Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotechnology, 26, 1269–1275. doi:10.1038/nbt.1502.
Tateishi, K., He, J., Taranova, O., Liang, G., D’Alessio, A., & Zhang, Y. (2008). Generation of insulin-secreting islet-like clusters from human skin fibroblasts. Journal of Biological Chemistry, 283, 31601–31607. doi:10.1074/jbc.M806597200.
Hanna, J., Wernig, M., Markoulaki, S., et al. (2007). Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science, 318, 1920–1923. doi:10.1126/science.1152092.
Wernig, M., Zhao, J. P., Pruszak, J., et al. (2008). Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America, 105, 5856–5861. doi:10.1073/pnas.0801677105.
Zhang D, Jiang W, Liu M, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Research 2009; In press.
Zhang, J., Wilson, G. F., Soerens, A. G., et al. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research, 104, e30–e41. doi:10.1161/CIRCRESAHA.108.192237.
Narazaki, G., Uosaki, H., Teranishi, M., et al. (2008). Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation, 118, 498–506. doi:10.1161/CIRCULATIONAHA.108.769562.
Xu, D., Alipio, Z., Fink, L. M., et al. (2009). Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proceedings of the National Academy of Sciences of the United States of America, 106, 808–813. doi:10.1073/pnas.0812090106.
Ebert, A. D., Yu, J., Rose, F. F., Jr., Mattis, V. B., Lorson, C. L., Thomson, J. A., et al. (2009). Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature, 457, 277–280. doi:10.1038/nature07677.
Blelloch, R., Venere, M., Yen, J., & Ramalho-Santos, M. (2007). Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell, 1, 245–247. doi:10.1016/j.stem.2007.08.008.
Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. iPS Cell Generation Using a Single Lentiviral Stem Cell Cassette. Stem Cells. 2008; In press.
Soldner, F., Hockemeyer, D., Beard, C., et al. (2009). Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell, 136, 964–977. doi:10.1016/j.cell.2009.02.013.
Carey, B. W., Markoulaki, S., Hanna, J., et al. (2009). Reprogramming of murine and human somatic cells using a single polycistronic vector. Proceedings of the National Academy of Sciences of the United States of America, 106, 157–162. doi:10.1073/pnas.0811426106.
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G., & Hochedlinger, K. (2008). Induced pluripotent stem cells generated without viral integration. Science, 322, 945–949. doi:10.1126/science.1162494.
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T., & Yamanaka, S. (2008). Generation of mouse induced pluripotent stem cells without viral vectors. Science, 322, 949–953. doi:10.1126/science.1164270.
Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature online 2009.
Yamanaka, S. (2009). A fresh look at iPS cells. Cell, 137, 13–17. doi:10.1016/j.cell.2009.03.034.
Zhou, H., Wu, S., Joo, J. Y., et al. (2009). Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell, 4, 381–384. doi:10.1016/j.stem.2009.04.005.
Ponzielli, R., Katz, S., Barsyte-Lovejoy, D., & Penn, L. Z. (2005). Cancer therapeutics: targeting the dark side of Myc. European Journal of Cancer, 41, 2485–2501. doi:10.1016/j.ejca.2005.08.017.
Aoi, T., Yae, K., Nakagawa, M., et al. (2008). Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 321, 699–702. doi:10.1126/science.1154884.
Wernig, M., Meissner, A., Cassady, J. P., & Jaenisch, R. (2008). c-Myc is dispensible for direct reprogramming of mouse fibroblasts. Cell Stem Cell, 2, 10–12. doi:10.1016/j.stem.2007.12.001.
Chen, Y., Shi, L., Zhang, L., et al. (2008). The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. Journal of Biological Chemistry, 283, 17969–17978. doi:10.1074/jbc.M802917200.
Trosko, J. E. (2006). From adult stem cells to cancer stem cells: Oct-4 Gene, cell-cell communication, and hormones during tumor promotion. Annals of the New York Academy of Sciences, 1089, 36–58. doi:10.1196/annals.1386.018.
Rowland, B. D., & Peeper, D. S. (2006). KLF4, p21 and context-dependent opposing forces in cancer. Nature Reviews Cancer, 6, 11–23. doi:10.1038/nrc1780.
Duinsbergen, D., Eriksson, M., 't Hoen, P. A., Frisén, J., & Mikkers, H. (2008). Induced pluripotency with endogenous and inducible genes. Experimental Cell Research, 314, 3255–3263. doi:10.1016/j.yexcr.2008.06.024.
Eminli, S., Utikal, J., Arnold, K., Jaenisch, R., & Hochedlinger, K. (2008). Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells, 26, 2467–2474. doi:10.1634/stemcells.2008-0317.
Shi, Y., Do, J. T., Desponts, C., Hahm, H. S., Schöler, H. R., & Ding, S. (2008). A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell, 2, 525–528. doi:10.1016/j.stem.2008.05.011.
Kim, J. B., Zaehres, H., Wu, G., et al. (2008). Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature, 454, 646–650. doi:10.1038/nature07061.
Silva, J., Barrandon, O., Nichols, J., Kawaguchi, J., Theunissen, T., & Smith, A. (2008). Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biology, 6, 2237–2247. doi:10.1371/journal.pbio.0060253.
Kim, J. B., Sebastiano, V., Wu, G., et al. (2009). Oct4-induced pluripotency in adult neural stem cells. Cell, 136, 411–419. doi:10.1016/j.cell.2009.01.023.
Qin, D., Gan, Y., Shao, K., et al. (2008). Mouse meningiocytes express sox2 and yield high efficiency of chimeras after nuclear reprogramming with exogenous factors. Journal of Biological Chemistry, 283, 33730–33735. doi:10.1074/jbc.M806788200.
Huangfu, D., Maehr, R., Guo, W., et al. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotechnology, 26, 795–797. doi:10.1038/nbt1418.
Marson, A., Foreman, R., Chevalier, B., et al. (2008). Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell, 3, 132–135. doi:10.1016/j.stem.2008.06.019.
Shi, Y., Desponts, C., Do, J. T., Hahm, H. S., Schöler, H. R., & Ding, S. (2008). Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell, 3, 568–574. doi:10.1016/j.stem.2008.10.004.
Mikkelsen, T. S., Hanna, J., Zhang, X., et al. (2008). Dissecting direct reprogramming through integrative genomic analysis. Nature, 454, 49–55. doi:10.1038/nature07056.
Pesce, M., & Scholer, H. R. (2001). Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells, 19, 271–27. doi:10.1634/stemcells.19-4-271.
Pesce, M., & Scholer, H. (2000). Oct4: control of totipotency and germline determination. Molecular Reproduction and Development, 55, 452–457. doi:10.1002/(SICI)1098-2795(200004)55:4<452::AID-MRD14>3.0.CO;2-S.
Gidekel, S., Pizov, G., Bergman, Y., & Pikarsky, E. (2003). Oct-3/4 is a dosedependent oncogenic fate determinant. Cancer Cell, 4, 361–370. doi:10.1016/S1535-6108(03)00270-8.
Niwa, H., Miyazaki, J., & Smith, A. G. (2000). Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature Genetics, 24, 372–376. doi:10.1038/74199.
Zaehres, H., Lensch, M. W., Daheron, L., Stewart, S. A., Itskovitz-Eldor, J., & Daley, G. Q. (2005). High-efficiency RNA interference in human embryonic stem cells. Stem Cells, 23, 299–305. doi:10.1634/stemcells.2004-0252.
Kehler, J., Tolkunova, E., Koschorz, B., et al. (2004). Oct4 is required for primordial germ cell survival. EMBO Reports, 5, 1078–1083. doi:10.1038/sj.embor.7400279.
Cheng, L., Sung, M. T., Cossu-Rocca, P., et al. (2007). OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. Journal of Pathology, 211, 1–9. doi:10.1002/path.2105.
Avilion, A. A., Nicolis, S. K., Pevny, L. H., Perez, L., Vivian, N., & Lovell-Badge, R. (2003). Multipotent cell lineages in early mouse development depend on SOX2 function. Genes and Development, 17, 126–140. doi:10.1101/gad.224503.
Miyagi, S., Saito, T., Mizutani, K., et al. (2004). The Sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Molecular and Cellular Biology, 24, 4207–4220. doi:10.1128/MCB.24.10.4207-4220.2004.
Yuan, H., Corbi, N., Basilico, C., & Dailey, L. (1995). Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes and Development, 9, 2635–2645. doi:10.1101/gad.9.21.2635.
Nishimoto, M., Miyagi, S., Katayanagi, T., Tomioka, M., Muramatsu, M., & Okuda, A. (2003). The embryonic Octamer factor 3/4 displays distinct DNA binding specificity from those of other Octamer factors. Biochemical and Biophysical Research Communications, 302, 581–586. doi:10.1016/S0006-291X(03)00218-3.
Masui, S., Nakatake, Y., Toyooka, Y., et al. (2007). Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nature Cell Biology, 9, 625–635. doi:10.1038/ncb1589.
Chew, J. L., Loh, Y. H., Wensheng, Z., et al. (2005). Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Molecular and Cellular Biology, 25, 6031–6046.
Ferri, A. L., Cavallaro, M., Braida, D., et al. (2004). Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development, 131, 3805–3819. doi:10.1242/dev.01204.
Williamson, K. A., Hever, A. M., Rainger, J., et al. (2006). Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Human Molecular Genetics, 15, 1413–1422. doi:10.1093/hmg/ddl064.
Kelberman, D., Rizzoti, K., Avilion, A., et al. (2006). Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitarygonadal axis in mice and humans. Journal of Clinical Investigation, 116, 2442–2455.
Dong, C., Wilhelm, D., & Koopman, P. (2004). Sox genes and cancer. Cytogenet Genome Research, 105, 442–447. doi:10.1159/000078217.
Dang, C. V., O’Donnell, K. A., Zeller, K. I., Nguyen, T., Osthus, R. C., & Li, F. (2006). The c-Myc target gene network. Seminars in Cancer Biology, 16, 253–264. doi:10.1016/j.semcancer.2006.07.014.
Dang, D. T., Pevsner, J., & Yang, V. W. (2000). The biology of the mammalian Kruppel-like family of transcription factors. International Journal of Biochemistry and Cell Biology, 32, 1103–1121. doi:10.1016/S1357-2725(00)00059-5.
Wei, D., Kanai, M., Huang, S., & Xie, K. (2006). Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis, 27, 23–31. doi:10.1093/carcin/bgi243.
Segre, J. A., Bauer, C., & Fuchs, E. (1999). Klf4 is a transcription factor required for establishing the barrier function of the skin. Nature Genetics, 22, 356–360. doi:10.1038/11926.
Conkright, M. D., Wani, M. A., Anderson, K. P., & Lingrel, J. B. (1999). A gene encoding an intestinal-enriched member of the Kruppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Research, 27, 1263–1270. doi:10.1093/nar/27.5.1263.
Garrett-Sinha, L. A., Eberspaecher, H., Seldin, M. F., & de Crombrugghe, B. (1996). A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. Journal of Biological Chemistry, 271, 31384–31390. doi:10.1074/jbc.271.49.31384.
Shields, J. M., Christy, R. J., & Yang, V. W. (1996). Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. Journal of Biological Chemistry, 271, 20009–20017. doi:10.1074/jbc.271.33.20009.
Nakatake, Y., Fukui, N., Iwamatsu, Y., et al. (2006). Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core promoter in embryonic stem cells. Molecular and Cellular Biology, 26, 72–7782. doi:10.1128/MCB.00468-06.
Jiang, J., Chan, Y. S., Loh, Y. H., et al. (2008). A core Klf circuitry regulates self-renewal of embryonic stem cells. Nature Cell Biology, 10, 353–360. doi:10.1038/ncb1698.
Chen, X., Johns, D. C., Geiman, D. E., et al. (2001). Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. Journal of Biological Chemistry, 276, 30423–30428. doi:10.1074/jbc.M101194200.
McConnell, B. B., Ghaleb, A. M., Nandan, M. O., & Yang, V. W. (2007). The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays, 29, 549–557. doi:10.1002/bies.20581.
Ohnishi, S., Ohnami, S., Laub, F., et al. (2003). Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochemical and Biophysical Research Communications, 308, 251–256. doi:10.1016/S0006-291X(03)01356-1.
Katz, J. P., Perreault, N., Goldstein, B. G., et al. (2005). Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology, 128, 935–945. doi:10.1053/j.gastro.2005.02.022.
Foster, K. W., Frost, A. R., McKie-Bell, P., et al. (2000). Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Research, 60, 6488–6495.
Wang, N., Liu, Z. H., Ding, F., et al. (2002). Downregulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World Journal of Gastroenterology, 8, 966–970.
Zhao, W., Hisamuddin, I. M., Nandan, M. O., et al. (2004). Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene, 23, 395–402. doi:10.1038/sj.onc.1207067.
Lebofsky, R., & Walter, J. C. (2007). New Myc-anisms for DNA replication and tumorigenesis? Cancer Cell, 12, 102–103. doi:10.1016/j.ccr.2007.07.013.
Patel, J. H., Loboda, A. P., Showe, M. K., Showe, L. C., & McMahon, S. B. (2004). Analysis of genomic targets reveals complex functions of MYC. Nature Reviews Cancer, 4, 562–568. doi:10.1038/nrc1393.
Cawley, S., Bekiranov, S., Ng, H. H., et al. (2004). Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell, 116, 499–509. doi:10.1016/S0092-8674(04)00127-8.
Cowling, V. H., & Cole, M. D. (2006). Mechanism of transcriptional activation by the Myc oncoproteins. Seminars in Cancer Biology, 16, 242–252. doi:10.1016/j.semcancer.2006.08.001.
Chang, T. C., Yu, D., Lee, Y. S., et al. (2008). Widespread microRNA repression by Myc contributes to tumorigenesis. Nature Genetics, 40, 43–50. doi:10.1038/ng.2007.30.
Davis, A. C., Wims, M., Spotts, G. D., Hann, S. R., & Bradley, A. (1993). A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes and Development, 7, 671–682. doi:10.1101/gad.7.4.671.
Baudino, T. A., McKay, C., Pendeville-Samain, H., et al. (2002). c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes and Development, 16, 2530–2543. doi:10.1101/gad.1024602.
Niwa, H. (2007). How is pluripotency determined and maintained? Development, 134, 635–646. doi:10.1242/dev.02787.
Chambers, I., Colby, D., Robertson, M., et al. (2003). Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell, 113, 643–655. doi:10.1016/S0092-8674(03)00392-1.
Chambers, I., Silva, J., Colby, D., et al. (2007). Nanog safeguards pluripotency and mediates germline development. Nature, 450, 1230–1234. doi:10.1038/nature06403.
Hyslop, L., Stojkovic, M., Armstrong, L., et al. (2005). Downregulation of nanog induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells, 23, 1035–1043. doi:10.1634/stemcells.2005-0080.
Bhattacharya, B., Miura, T., Brandenberger, R., et al. (2004). Gene expression in human embryonic stem cell lines: unique molecular signature. Blood, 103, 2956–2964. doi:10.1182/blood-2003-09-3314.
Pan, G., Li, J., Zhou, Y., Zheng, H., & Pei, D. (2006). A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB Journal, 20, 1730–1732. doi:10.1096/fj.05-5543fje.
Loh, Y. H., Wu, Q., Chew, J. L., et al. (2006). The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genetics, 38, 431–440. doi:10.1038/ng1760.
Boyer, L. A., Lee, T. I., Cole, M. F., et al. (2005). Core transcriptional regulatory circuitry in human embryonic stem cells. Cell, 122, 947–956. doi:10.1016/j.cell.2005.08.020.
Alon, U. (2007). Network motifs: theory and experimental approaches. Nature Reviews Genetics., 8, 450–461. doi:10.1038/nrg2102.
McAdams, H. H., & Arkin, A. (1997). Stochastic mechanisms in gene expression. Proceedings of the National Academy of Sciences of the United States of America, 94, 814–819. doi:10.1073/pnas.94.3.814.
Rosenfeld, N., Elowitz, M. B., & Alon, U. (2002). Negative autoregulation speeds the response times of transcription networks. Journal of Molecular Biology, 323, 785–793. doi:10.1016/S0022-2836(02)00994-4.
Kuroda, T., Tada, M., Kubota, H., et al. (2005). Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Molecular and Cellular Biology, 25, 2475–2485. doi:10.1128/MCB.25.6.2475-2485.2005.
Okumura-Nakanishi, S., Saito, M., Niwa, H., & Ishikawa, F. (2005). Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. Journal of Biological Chemistry, 280, 5307–5317. doi:10.1074/jbc.M410015200.
Rodda, D. J., Chew, J. L., Lim, L. H., et al. (2005). Transcriptional regulation of Nanog by OCT4 and SOX2. Journal of Biological Chemistry, 280, 24731–24737. doi:10.1074/jbc.M502573200.
Wang, J., Rao, S., Chu, J., et al. (2006). A protein interaction network for pluripotency of embryonic stem cells. Nature, 444, 364–368. doi:10.1038/nature05284.
Feldman, N., Gerson, A., Fang, J., et al. (2006). G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nature Cell Biology, 8, 188–194. doi:10.1038/ncb1353.
Bernstein, B. E., Mikkelsen, T. S., Xie, X., et al. (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell, 125, 315–326. doi:10.1016/j.cell.2006.02.041.
Azuara, V., Perry, P., Sauer, S., et al. (2006). Chromatin signatures of pluripotent cell lines. Nature Cell Biology, 8, 532–538. doi:10.1038/ncb1403.
Guenther, M. G., Levine, S. S., Boyer, L. A., Jaenisch, R., & Young, R. A. (2007). A chromatin landmark and transcription initiation at most promoters in human cells. Cell, 130, 77–88. doi:10.1016/j.cell.2007.05.042.
Boyer, L. A., Plath, K., Zeitlinger, J., et al. (2006). Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature, 441, 349–353. doi:10.1038/nature04733.
Silva, J., & Smith, A. (2008). Capturing pluripotency. Cell, 132, 532–536. doi:10.1016/j.cell.2008.02.006.
Smith, A. G., Heath, J. K., Donaldson, D. D., et al. (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature, 336, 688–690. doi:10.1038/336688a0.
Niwa, H., Burdon, T., Chambers, I., & Smith, A. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes and Development, 12, 2048–2060. doi:10.1101/gad.12.13.2048.
Liu, Y., Ji, L., Ten, Y., Wang, Y., & Pei, X. (2007). The molecular mechanism of embryonic stem cell pluripotency and self-renewal. Science in China Series C Life Sciences, 50, 619–623. doi:10.1007/s11427-007-0074-5.
Xu, R. H., Chen, X., Li, D. S., et al. (2002). BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nature Biotechnology, 20, 1261–1264. doi:10.1038/nbt761.
Qi, X., Li, T. G., Hao, J., et al. (2004). BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proceedings of the National Academy of Sciences of the United States of America, 101, 6027–6032. doi:10.1073/pnas.0401367101.
Jaenisch, R., & Young, R. (2008). Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell, 132, 567–582. doi:10.1016/j.cell.2008.01.015.
Meissner, A., Wernig, M., & Jaenisch, R. (2007). Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nature Biotechnology, 25, 1177–1181. doi:10.1038/nbt1335.
Hotta, A., & Ellis, J. (2008). Retroviral vector silencing during iPS cell induction: an epigenetic beacon that signals distinct pluripotent states. Journal of Cellular Biochemistry, 105, 940–948. doi:10.1002/jcb.21912.
McMahon, S. B., Van Buskirk, H. A., Dugan, K. A., Copeland, T. D., & Cole, M. D. (1998). The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell, 94, 363–374. doi:10.1016/S0092-8674(00)81479-8.
Vervoorts, J., Luscher-Firzlaff, J. M., Rottmann, S., et al. (2003). Stimulation of c-MYC transcriptional activity and acetylation by recruitment of the cofactor CBP. EMBO Reports, 4, 484–490. doi:10.1038/sj.embor.embor821.
Knoepfler, P. S., Zhang, X. Y., Cheng, P. F., et al. (2006). Myc influences global chromatin structure. EMBO Journal, 25, 2723–2734. doi:10.1038/sj.emboj.7601152.
Yamanaka, S. (2007). Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell, 1, 39–49. doi:10.1016/j.stem.2007.05.012.
Hooker, C. W., & Hurlin, P. J. (2006). Of Myc and Mnt. Journal of Cell Science, 119, 208–216. doi:10.1242/jcs.02815.
Welstead, G. G., Schorderet, P., & Boyer, L. A. (2008). The reprogramming language of pluripotency. Current Opinion in Genetics and Development, 18, 123–129. doi:10.1016/j.gde.2008.01.013.
Egli, D., Birkhoff, G., & Eggan, K. (2008). Mediators of reprogramming: transcription factors and transitions through mitosis. Nature Reviews Molecular Cell Biology, 9, 505–516. doi:10.1038/nrm2439.
Dominguez-Sola, D., Ying, C. Y., Grandori, C., et al. (2007). Nontranscriptional control of DNA replication by c-Myc. Nature, 448, 445–451. doi:10.1038/nature05953.
Wu, K. J., Grandori, C., Amacker, M., et al. (1999). Direct activation of TERT transcription by c-MYC. Nature Genetics, 21, 220–224. doi:10.1038/6010.
Rowland, B. D., Bernards, R., & Peeper, D. S. (2005). The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nature Cell Biology, 7, 1074–1082. doi:10.1038/ncb1314.
Zhang, W., Geiman, D. E., Shields, J. M., et al. (2000). The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. Journal of Biological Chemistry, 275, 18391–18398. doi:10.1074/jbc.C000062200.
Seoane, J., Le, H. V., & Massague, J. (2002). Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature, 419, 729–734. doi:10.1038/nature01119.
Lin, T., Chao, C., Saito, S., et al. (2005). p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nature Cell Biology, 7, 165–171. doi:10.1038/ncb1211.
Evans, P. M., Zhang, W., Chen, X., et al. (2007). Krurppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. Journal of Biological Chemistry, 282, 33994–34002. doi:10.1074/jbc.M701847200.
Balzer, E., & Moss, E. G. (2007). Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biology, 4, 16–25.
Yu, F., Yao, H., Zhu, P., et al. (2007). let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell, 131, 1109–1123. doi:10.1016/j.cell.2007.10.054.
Kumar, M. S., Erkeland, S. J., Pester, R. E., et al. (2008). Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proceedings of the National Academy of Sciences of the United States of America, 105, 3903–3908. doi:10.1073/pnas.0712321105.
Jackson-Grusby, L., Beard, C., Possemato, R., et al. (2001). Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nature Genetics, 27, 31–39. doi:10.1038/83730.
Loh, Y. H., Zhang, W., Chen, X., George, J., & Ng, H. H. (2007). Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes and Development, 21, 2545–2557. doi:10.1101/gad.1588207.
Stadtfeld, M., Brennand, K., & Hochedlinger, K. (2008). Reprogramming of pancreatic β cells into induced pluripotent stem cells. Current Biology, 18, 890–894. doi:10.1016/j.cub.2008.05.010.
Verfaillie, C. (2008). The undoing of differentiation by four defined factors: a big step forward towards generating patient specific pluripotent stem cells. Journal of Hepatology, 49, 876–878. doi:10.1016/j.jhep. 2008.08.007.
Wilmut, I. (2007). The first direct reprogramming of adult human fibroblasts. Cell Stem Cell, 1, 3–594. doi:10.1016/j.stem.2007.11.013.
Varas F, Stadtfeld M, De Andres-Aguayo L, et al. Fibroblast derived induced pluripotent stem cells show no common retroviral vector insertions. Stem Cells. 2008; In press.
Gregory, M. A., Qi, Y., & Hann, S. R. (2005). The ARF tumor suppressor: keeping Myc on a leash. Cell Cycle, 4, 249–252.
Brambrink, T., Foreman, R., Welstead, G. G., et al. (2008). Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell, 2, 151–159. doi:10.1016/j.stem.2008.01.004.
Stadtfeld, M., Maherali, N., Breault, D., & Hochedlinger, K. (2008). Defining Molecular Cornerstones during Fibroblast to iPS Cell Reprogramming in Mouse. Cell Stem Cell, 2, 230–240. doi:10.1016/j.stem.2008.02.001.
Ruau, D., Ensenat-Waser, R., Dinger, T. C., et al. (2008). Pluripotency associated genes are reactivated by chromatin-modifying agents in neurosphere cells. Stem Cells, 26, 920–926. doi:10.1634/stemcells.2007-0649.
Cyranoski, D. (2008). 5 things to know before jumping on the iPS bandwagon. Nature, 452, 406–408. doi:10.1038/452406a.
Hanna, J., Markoulaki, S., Schorderet, P., et al. (2008). Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell, 133, 250–264. doi:10.1016/j.cell.2008.03.028.
Boyes, J., & Bird, A. (1992). Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO Journal, 11, 327–333.
Lorincz, M. C., Schubeler, D., Hutchinson, S. R., Dickerson, D. R., & Groudine, M. (2002). DNA methylation density influences the stability of an epigenetic imprint and Dnmt3a/b-independent de novo methylation. Molecular and Cellular Biology, 22, 7572–7580. doi:10.1128/MCB.22.21.7572-7580.2002.
Sridharan, R., Tchieu, J., Mason, M. J., et al. (2009). Role of the murine reprogramming factors in the induction of pluripotency. Cell, 136, 364–77. doi:10.1016/j.cell.2009.01.001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheper, W., Copray, S. The Molecular Mechanism of Induced Pluripotency: A Two-Stage Switch. Stem Cell Rev and Rep 5, 204–223 (2009). https://doi.org/10.1007/s12015-009-9077-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-009-9077-x