Abstract
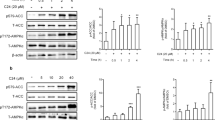
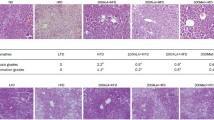
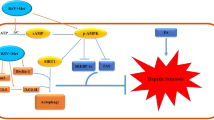
Metformin improves lipid profile, however, combination therapy is developing to increase its effectiveness and reduce the deleterious effects of metformin. Chlorogenic acid (CGA) has exhibited lipid-lowering effects. This study aimed to investigate the combined effect of metformin and CGA on lipid accumulation, as well as to elucidate the engaged mechanism in HepG2 cells. To find the non-lethal doses of metformin and CGA, MTT assay was performed. High Glucose (HG) at 33 mM was used to induce lipogenesis in HepG2 cells. Following treatment with different concentrations of metformin and CGA, total lipid content (Oil Red O-staining), triglyceride level, the genes expression of SREBP-1c and FAS, and phosphorylation of AMPK and ACC were measured. Both Metformin and CGA decreased HG-induced lipid accumulation individually, by decreasing total lipid content and triglyceride level. The lowest effective doses of metformin and CGA were 0.25 mM and 5 μM, respectively, which significantly reduced SREBP-1c and FAS genes expression. The combination of these concentrations reinforced these effects. The phosphorylation of AMPK and ACC were more increased by metformin in combination with CGA than both individually. Our findings suggest that CGA synergistically enhances metformin lipid reducing action via the regulating of involved factors in fatty acid synthesis. Therefore, co-administration of metformin with CGA may have further medical value in treating lipid metabolism disorders.





Similar content being viewed by others
Abbreviations
- ACC:
-
acetyl-CoA carboxylase
- cDNA:
-
Complementary DNA
- CGA:
-
Chlorogenic acid
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- FBS:
-
fetal bovine serum
- HG:
-
high concentration of D-glucose
- IC50:
-
inhibitory concentration values
- ME:
-
median effective
- NAFLD:
-
non-alcoholic fatty liver disease
- NG:
-
normal glucose
- SREBP-1c:
-
sterol regulatory element-binding protein-1c
- T2DM:
-
Type 2 diabetes mellitus
References
Cho, N., Shaw, J., Karuranga, S., Huang, Y. D., da Rocha Fernandes, J. & Ohlrogge, A., et al. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract, 138, 271–281.
Marín-Peñalver, J. J., Martín-Timón, I., Sevillano-Collantes, C., & del Cañizo-Gómez, F. J. (2016). Update on the treatment of type 2 diabetes mellitus. World Journal of Diabetes, 7(17), 354.
Zheng, Y., Ley, S. H., & Hu, F. B. (2018). Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology, 14(2), 88.
Richard, J., & Lingvay, I. (2011). Hepatic steatosis and Type 2 diabetes: current and future treatment considerations. Expert Review of Cardiovascular Therapy, 9(3), 321–328.
Dai, W., Ye, L., Liu, A., Wen, S. W., Deng, J. & Wu, X., et al. (2017). Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine, 96(39), e8179.
Ferre, P., & Foufelle, F. (2010). Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP‐1c. Diabetes, Obesity and Metabolism, 12, 83–92.
Shimomura, I., Matsuda, M., Hammer, R. E., Bashmakov, Y., Brown, M. S., & Goldstein, J. L. (2000). Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Molecular Cell, 6(1), 77–86.
Cornier, M.-A., Dabelea, D., Hernandez, T. L., Lindstrom, R. C., Steig, A. J., & Stob, N. R., et al. (2008). The metabolic syndrome. Endocrine Reviews, 29(7), 777–822.
Saponaro, C., Gaggini, M., Carli, F., & Gastaldelli, A. (2015). The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients., 7(11), 9453–9474.
Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., & Fenyk-Melody, J., et al. (2001). Role of AMP-activated protein kinase in mechanism of metformin action. The. Journal of Clinical Investigation, 108(8), 1167–1174.
Inzucchi, S. E., Bergenstal, R. M., Buse, J. B., Diamant, M., Ferrannini, E., & Nauck, M., et al. (2015). Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care, 38(1), 140–149.
Phung, O. J., Sobieraj, D. M., Engel, S. S., & Rajpathak, S. N. (2014). Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta‐analysis. Diabetes, Obesity and Metabolism, 16(5), 410–417.
Salpeter, S. R., Greyber, E., Pasternak, G. A., Salpeter, E. E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2010(4).
Song, R. (2016). Mechanism of metformin: a tale of two sites. Diabetes Care, 39(2), 187–189.
Stage, T. B., Brøsen, K., & Christensen, M. M. H. (2015). A comprehensive review of drug–drug interactions with metformin. Clinical Pharmacokinetics, 54(8), 811–824.
Thounaojam, M. C., Nammi, S., Jadeja, R. Natural products for the treatment of obesity, metabolic syndrome, and type 2 diabetes 2014. Hindawi; 2015, Published Special Issues.
Xia, X., & Weng, J. (2010). Targeting metabolic syndrome: candidate natural agents. Journal of Diabetes, 2(4), 243–249.
Chang, E., & Kim, C. Y. (2019). Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules., 24(6), 1157.
Shokri Afra, H., Zangooei, M., Meshkani, R., Ghahremani, M. H., Ilbeigi, D., & Khedri, A., et al. (2019). Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J Biochem Physiol, 75(2), 125–33.
Wang, S., Moustaid-Moussa, N., Chen, L., Mo, H., Shastri, A., & Su, R., et al. (2014). Novel insights of dietary polyphenols and obesity. The Journal of Nutritional Biochemistry, 25(1), 1–18.
Santana-Gálvez, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017). Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules., 22(3), 358.
de Sotillo, D. V. R., & Hadley, M. (2002). Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. The Journal of Nutritional Biochemistry, 13(12), 717–726.
Nicasio, P., Aguilar‐Santamaría, L., Aranda, E., Ortiz, S., & González, M. (2005). Hypoglycemic effect and chlorogenic acid content in two Cecropia species. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 19(8), 661–664.
Naveed, M., Hejazi, V., Abbas, M., Kamboh, A. A., Khan, G. J., & Shumzaid, M., et al. (2018). Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomedicine & Pharmacotherapy, 97, 67–74.
Meng S., Cao J., Feng Q., Peng J., Hu Y., eCAM am. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. 2013;2013.
Lin, W. Y., Xaiver Pi‐Sunyer, F., Chen, C. C., Davidson, L. E., Liu, C. S., & Li, T. C., et al. (2011). Coffee consumption is inversely associated with type 2 diabetes in Chinese. European Journal of Clinical Investigation, 41(6), 659–666.
van Dam, R. M. (2008). Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer. Applied Physiology, Nutrition, and Metabolism, 33(6), 1269–1283.
Baur, J. A. (2010). Biochemical effects of SIRT1 activators. Biochimica et Biophysica Acta (Bba)-Proteins and Proteomics., 1804(8), 1626–1634.
Chou, T.-C., & Talalay, P. (1984). Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation, 22, 27–55.
Osório, J. (2015). Hepatic lipogenesis independent of insulin in type 2 diabetes mellitus—a paradox clarified. Nature Reviews Endocrinology., 11(3), 130-.
Donnelly, K. L., Smith, C. I., Schwarzenberg, S. J., Jessurun, J., Boldt, M. D., & Parks, E. J. (2005). Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. The Journal of Clinical Investigation, 115(5), 1343–1351.
Gorgani-Firuzjaee, S., & Meshkani, R. (2015). SH2 domain-containing inositol 5-phosphatase (SHIP2) inhibition ameliorates high glucose-induced de-novo lipogenesis and VLDL production through regulating AMPK/mTOR/SREBP1 pathway and ROS production in HepG2 cells. Free Radical Biology and Medicine, 89, 679–689.
Li, Y., Xu, S., Mihaylova, M. M., Zheng, B., Hou, X., & Jiang, B., et al. (2011). AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabolism, 13(4), 376–388.
Zang, M., Zuccollo, A., Hou, X., Nagata, D., Walsh, K., & Herscovitz, H., et al. (2004). AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. Journal of Biological Chemistry, 279(46), 47898–47905.
Ong, K. W., Hsu, A., & Tan, B. K. H. (2013). Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochemical Pharmacology, 85(9), 1341–1351.
Wang, Q., Liu, S., Zhai, A., Zhang, B., & Tian, G. (2018). AMPK-mediated regulation of lipid metabolism by phosphorylation. Biological and Pharmaceutical Bulletin, 41(7), 985–993.
Cheang, W. S., Tian, X. Y., Wong, W. T., Lau, C. W., Lee, S. S.-T., & Chen, Z. Y., et al. (2014). Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate–activated protein kinase–peroxisome proliferator–activated receptor δ pathway. Arteriosclerosis, Thrombosis, And Vascular Biology, 34(4), 830–836.
Foretz, M., Hébrard, S., Leclerc, J., Zarrinpashneh, E., Soty, M., & Mithieux, G., et al. (2010). Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. The. Journal of Clinical Investigation, 120(7), 2355–2369.
El-Mir, M.-Y., Nogueira, V., Fontaine, E., Avéret, N., Rigoulet, M., & Leverve, X. (2000). Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. Journal of Biological Chemistry, 275(1), 223–228.
Sudeep, H., Venkatakrishna, K., Patel, D., & Shyamprasad, K. (2016). Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complementary and Alternative Medicine, 16(1), 1–5.
Mubarak, A., Hodgson, J. M., Considine, M. J., Croft, K. D., & Matthews, V. B. (2013). Supplementation of a high-fat diet with chlorogenic acid is associated with insulin resistance and hepatic lipid accumulation in mice. Journal of Agricultural and Food Chemistry, 61(18), 4371–4378.
Tsuda, S., Egawa, T., Ma, X., Oshima, R., Kurogi, E., & Hayashi, T. (2012). Coffee polyphenol caffeic acid but not chlorogenic acid increases 5′ AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. The Journal of Nutritional Biochemistry, 23(11), 1403–1409.
Murase, T., Misawa, K., Minegishi, Y., Aoki, M., Ominami, H., & Suzuki, Y., et al. (2011). Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. American Journal of Physiology-Endocrinology and Metabolism, 300(1), E122–E33.
Zamani‐Garmsiri, F., Ghasempour, G., Aliabadi, M., Hashemnia, S. M. R., Emamgholipour, S., & Meshkani, R. (2021). Combination of metformin and chlorogenic acid attenuates hepatic steatosis and inflammation in high‐fat diet fed mice. IUBMB Life, 73(1), 252–263.
Acknowledgements
This work was financially supported by a grant (96-01-30-34043) from the Deputy of Research, Tehran University of Medical Sciences. The results described in this paper were part of the student thesis. We acknowledge the Department of Clinical Biochemistry of Tehran University of Medical Sciences for supporting during the execution of this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Namvarjah, F., Shokri-Afra, H., Moradi-Sardareh, H. et al. Chlorogenic acid improves anti-lipogenic activity of metformin by positive regulating of AMPK signaling in HepG2 cells. Cell Biochem Biophys 80, 537–545 (2022). https://doi.org/10.1007/s12013-022-01077-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01077-1