Abstract
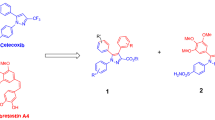
Herein, we report the synthesis, characterization and anticancer activity of six novel complexes of non-steroidal anti-inflammatory drug niflumic acid with Co(II) and Ni(II). In vitro cytotoxicity screening in MCF-7, HepG2 and HT-29 cancer cell lines showed that the complex 3 [Co(nif)2(met)(4-pic)] and complex 6 [Ni(nif)2(met)(4-pic)] among all the complexes exhibited the highest cytotoxicity against MCF-7 cells with IC50 values of 11.14 µM and, 41.47 µM, respectively. Besides, all the complexes exhibited significantly higher selectivity towards mouse fibroblast 3T3L1 cells. Further mechanistic studies with both complexes on MCF-7 cells revealed their cytotoxic action through the mitochondrial-dependent apoptotic pathway causing an increase oxidative/nitrosative stress, decrease in mitochondrial membrane potential (ΔΨm), inducing the multicaspase activation and arresting the cell cycle at S phase. q-PCR analysis resulted in an increase in the expression of the apoptotic marker proteins bax, p53 and caspase-3 and -8 in MCF-7 cells, but a decrease in the expression of antiapoptotic bcl-2 gene. Moreover, both complexes induced the apoptosis through the inhibition of PI3K/Akt signaling pathway by decreasing the expression of PI3K and increasing dephosphorylation form of Akt protein. These results provide a significant contribution to the explanation of the anticancer mechanisms of these complexes in MCF-7 cells.

Highlights
-
Novel Co(II) and Ni(II) complexes were synthesized and characterized by FT-IR, elemental and thermal analysis techniques.
-
Complex 3 showed the highest cytotoxic activity against MCF-7, HT-29, and HepG2 cells.
-
Complex 3 and 6 triggered the apoptosis by inducing oxidative stress in MCF-7 cells.
-
Complex 3 and 6 deactivated the PI3K/Akt pathway in MCF-7 cells.
-
Complex 3 and 6 modulated the apoptotic marker genes: bax, bcl-2, p53, caspase-3 and -8 in MCF-7 cells.







Similar content being viewed by others
References
Rosenberg, B. (1980). Cisplatin: its history and possible mechanisms of action. Academic Press: Inc. https://doi.org/10.1016/b978-0-12-565050-2.50006-1.
Benedetti, B. T., Peterson, E. J., Kabolizadeh, P., Martínez, A., Kipping, R., & Farrell, N. P. (2011). Effects of noncovalent platinum drug-protein interactions on drug efficacy: use of fluorescent conjugates as probes for drug metabolism. Molecular Pharmaceutics, 8, 940–948. https://doi.org/10.1021/mp2000583.
Bhargava, A., & Vaishampayan, U. N. (2009). Satraplatin: leading the new generation of oral platinum agents. Expert Opinion on Investigational Drugs, 18, 1787–1797. https://doi.org/10.1517/13543780903362437.
Frezza, M., Hindo, S., Chen, D., Davenport, A., Schmitt, S., Tomco, D., & Ping Dou, Q. (2010). Novel metals and metal complexes as platforms for cancer therapy. Current Pharmaceutical Design, 16, 1813–1825. https://doi.org/10.2174/138161210791209009.
Karlenius, T. C., & Tonissen, K. F. (2010). Thioredoxin and cancer: a role for thioredoxin in all states of tumor oxygenation. Cancers (Basel), 2, 209–232. https://doi.org/10.3390/cancers2020209.
Bindoli, A., Pia, M., Scutari, G., Gabbiani, C., Casini, A., Messori, L., Thioredoxin reductase: a target for gold compounds acting as potential anticancer drugs, 253 (2009) 1692–1707. https://doi.org/10.1016/j.ccr.2009.02.026.
Gupte, A., & Mumper, R. J. (2009). Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treatment Reviews, 35, 32–46. https://doi.org/10.1016/j.ctrv.2008.07.004.
Monneret, C. (2011). Platinum anticancer drugs. From serendipity to. Annales Pharmaceutiques Françaises, 69, 286–295. https://doi.org/10.1016/j.pharma.2011.10.001.
Johnstone, T. C., Suntharalingam, K., Lippard, S. J., The next generation of platinum drugs: targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs, (2016). https://doi.org/10.1021/acs.chemrev.5b00597.
Kumazawa, S., Hamasaka, T., & Nakayama, T. (2004). Antioxidant activity of propolis of various geographic origins. Food Chemistry, 84, 329–339.
Mi, Q., Ma, Y., Gao, X., Liu, R., Liu, P., Mi, Y., Fu, X., 2-Deoxyglucose conjugated platinum (II) complexes for targeted therapy: design, synthesis, and antitumor activity, 1102 (2016). https://doi.org/10.1080/07391102.2015.1114972.
Du, C. P. V., Elliott, C. J., Connor, R. A. O., Heenan, M. M., Coyle, S., Cleary, I. M., Kavanagh, K., Verhaegen, S., Loughlin, C. M. O., Nicamhlaoibh, R., & Clynes, M. (1998). Original paper enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory Drugs (NSAIDs). European Journal of Cancer, 34, 1250–1259.
Zianna, A., Ristovic, M. S., Psomas, G., Hatzidimitriou, A., Coutouli-Argyropoulou, E., & Lalia-Kantouri, M. (2015). Cadmium(II) complexes of 5-nitro-salicylaldehyde and α-diimines: synthesis, structure and interaction with calf-thymus DNA. Journal of Coordination Chemistry, 68, 4444–4463. https://doi.org/10.1080/00958972.2015.1101075.
Zampakou, M., Hatzidimitriou, A. G., Athanasios, N., & Psomas, G. (2015). Neutral and cationic manganese (II)–diclofenac complexes: structure and biological evaluation. Journal of Coordination Chemistry, 68, 4355–4372. https://doi.org/10.1080/00958972.2015.1098633.
Johnsen, J. I., Lindskog, M., Ponthan, F., Pettersen, I. (2005) NSAIDs in neuroblastoma therapy, 228, 195–201. https://doi.org/10.1016/j.canlet.2005.01.058.
Amin, A. R., Vyas, P., Atrur, M., Leszczynska-pizlak, J., Patelt, I. R., Weissmann, G., & Abramson, S. B. (1995). The mode of action of aspirin-like drugs: effect on inducible nitric oxide synthase. Medical Sciences, 92, 7926–7930.
Rao, P. N. P., Knaus, E. E., Road, T. P., & Jolla, L. (2008). Evolution of nonsteroidal anti-inflammatory cyclooxygenase (COX) inhibition and beyond drugs (NSAIDs). Journal of Pharmaceutical Sciences, 11, 81–110.
Basha, R., Ahmad, S., Safe, S., & Abbruzzese, J. L. (2011). Therapeutic applications of NSAIDS in cancer: special emphasis on tolfenamic acid. Frontiers in Bioscience-Scholar, 3, 797–805. https://doi.org/10.2741/S188.
Gasparini, L., Ongini, E., Wenk, G., Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer ’ s disease: old and new mechanisms of action, (2004) 521–536. https://doi.org/10.1111/j.1471-4159.2004.02743.x.
Ho Woo, D., Han, I. S., & Jung, G. (2004). Mefenamic acid-induced apoptosis in human liver cancer cell-lines through caspase-3 pathway. Life Sciences., 75, 2439–2449. https://doi.org/10.1016/j.lfs.2004.04.042.
Tarushi, A., Kara, Z., Kljun, J., Turel, I., Psomas, G., Papadopoulos, A. N., Kessissoglou, D. P., Antioxidant capacity and DNA-interaction studies of zinc complexes with a non-steroidal anti-inflammatory drug, mefenamic acid, 128 (2013) 85–96. https://doi.org/10.1016/j.jinorgbio.2013.07.013.
Tsiliki, P., Perdih, F., Turel, I., & Psomas, G. (2013). Structure, DNA- and albumin-binding of the manganese (II) complex with the non-steroidal antiinflammatory drug niflumic acid. Polyhedron., 53, 215–222. https://doi.org/10.1016/j.poly.2013.01.049.
Palacios-hernández, T., Höp, H., Sánchez-salas, J. L., González-vergara, E., & Pérez-benítez, A. (2014). In vitro antibacterial activity of meclofenamate metal complexes with Cd (II) Pb (II), Co (II), and Cu (II). Crystal Structures of,139, 85–92. https://doi.org/10.1016/j.jinorgbio.2014.06.008.
Ma, Z., & Moulton, B. (2011). Recent advances of discrete coordination complexes and coordination polymers in drug delivery. Coordination Chemistry Reviews, 255, 1623–1641. https://doi.org/10.1016/j.ccr.2011.01.031.
Knauf, P. A., & Mann, N. A. (1984). Use of niflumic acid to determine the nature of the asymmetry of the human erythrocyte anion exchange system. Journal of General Physiology, 83, 703–725. https://doi.org/10.1085/jgp.83.5.703.
Sinkkonen, S., Mansikkamaki, S., Möykkynen, T., & Lüddens, H. (2003). Receptor subtype-dependent positive and negative modulation of GABAA receptor function by niflumic acid, a nonsteroidal anti-inflammatory drug. Molecular Pharmacology, 64, 753–763.
Balderas, E., Ateaga-tlecuitl, R., Rivera, M., Gomora, J. C., Darszon, A., Niflumic acid blocks native and recombinant T-type channels, (n.d.). https://doi.org/10.1002/jcp.22992.
Kumar, S., Pal, R., Venugopalan, P., Ferretti, V., & Tarpin, M. (2019). Inorganica chimica acta new copper (II) niflumate complexes with N-donor ligands: synthesis, characterization and evaluation of anticancer potential against human cell lines. Inorganica Chimica Acta, 488, 260–268. https://doi.org/10.1016/j.ica.2019.01.020.
Kim, B. M., Maeng, K., Lee, K. H., & Hong, S. H. (2011). Combined treatment with the Cox-2 inhibitor niflumic acid and PPARγ ligand ciglitazone induces ER stress/caspase-8-mediated apoptosis in human lung cancer cells. Cancer Letters, 300, 134–144. https://doi.org/10.1016/j.canlet.2010.09.014.
Smolko, L., Smolková, R., Samoľová, E., Morgan, I., Saoud, M., & Kaluđerović, G. N. (2020). Two isostructural Co (II) flufenamato and niflumato complexes with bathocuproine: analogues with a different cytotoxic activity. Journal of Inorganic Biochemistry., 210, 111160 https://doi.org/10.1016/j.jinorgbio.2020.111160.
Tarushi, A., Raptopoulou, C. P., Psycharis, V., Kessissoglou, D. P., Papadopoulos, A. N., Psomas, G., Interaction of zinc (II) with the non-steroidal anti-in fl ammatory drug niflumic acid, 176 (2017) 100–112. https://doi.org/10.1016/j.jinorgbio.2017.08.022.
Altay, A., Caglar, S., & Caglar, B. (2019). Silver(I) complexes containing diclofenac and niflumic acid induce apoptosis in human-derived cancer cell lines. Archives of Physiology and Biochemistry, 0, 1–11. https://doi.org/10.1080/13813455.2019.1662454.
Kefala, L., Hatzidimitriou, A. G., Kessissoglou, D. P., Perdih, F., Cobalt (II) complexes with non-steroidal anti-inflammatory drugs and α -diimines, 160 (2016) 125–139. https://doi.org/10.1016/j.jinorgbio.2015.12.015.
Skyrianou, K. C., Efthimiadou, E. K., Psycharis, V., Terzis, A., Kessissoglou, D. P., & Psomas, G. (2009). Nickel – quinolones interaction . Part 1 – Nickel (II) complexes with the antibacterial drug sparfloxacin: Structure and biological properties. Journal of Inorganic Biochemistry, 103, 1617–1625. https://doi.org/10.1016/j.jinorgbio.2009.08.011.
Ramírez-macías, I., Maldonado, C. R., Marín, C., Olmo, F., Gutiérrez-sánchez, R., Rosales, M. J., Quirós, M., Salas, J. M., & Sánchez-moreno, M. (2012). In vitro anti-leishmania evaluation of nickel complexes with a triazolopyrimidine derivative against Leishmania infantum and Leishmania braziliensis. Journal of Inorganic Biochemistry, 112, 1–9. https://doi.org/10.1016/j.jinorgbio.2012.02.025.
Skyrianou, K. C., Perdih, F., Papadopoulos, A. N., Turel, I., Kessissoglou, D. P., & Psomas, G. (2011). Nickel—quinolones interaction Part 5—Biological evaluation of nickel (II) complexes with fi rst-, second- and third-generation quinolones (A). Journal of Inorganic Biochemistry, 105, 1273–1285. https://doi.org/10.1016/j.jinorgbio.2011.06.005.
Dimiza, F., Papadopoulos, A. N., Tangoulis, V., Psycharis, V., Raptopoulou, C. P., Kessissoglou, D. P., & Psomas, G. (2012). Biological evaluation of cobalt (II) complexes with non-steroidal anti-in fl ammatory drug naproxen. Journal of Inorganic Biochemistry, 107, 54–64. https://doi.org/10.1016/j.jinorgbio.2011.10.014.
London, M. E., Lo, H., Gracia-mora, I., Poblano-mele, I., Barba-behrens, N., Synthesis, structure and biological activities of cobalt (II) and zinc (II) coordination compounds with 2-benzimidazole derivatives, 102 (2008) 1267–1276. https://doi.org/10.1016/j.jinorgbio.2008.01.016.
Bisceglie, F., Pinelli, S., Alinovi, R., Goldoni, M., Mutti, A., Camerini, A., Piola, L., Tarasconi, P., & Pelosi, G. (2014). Cinnamaldehyde and cuminaldehyde thiosemicarbazones and their copper (II) and nickel (II) complexes: A study to understand their biological activity. Journal of Inorganic Biochemistry, 140, 111–125. https://doi.org/10.1016/j.jinorgbio.2014.07.014.
Prabu, R., Vijayaraj, A., Suresh, R., Senbhagaraman, R., Kaviyarasan, V., Biological studies of macrobicyclic binuclear nickel (II) complexes of 1, 8-difunctionalized cyclam derivatives, 8972 (2013). https://doi.org/10.1080/00958972.2012.751488.
Ii, N., Jin, Y., Lewis, M. A., Gokhale, N. H., Long, E. C., & Cowan, J. A. (2007). Influence of Stereochemistry and Redox Potentials on the Single- and Double-Strand DNA Cleavage Efficiency of Cu(II)· and Ni(II)·Lys-Gly-His-Derived ATCUN Metallopeptides. American Chemical Society, 129, 8353–8361. https://doi.org/10.1021/ja0705083.
Barone, G., Gambino, N., Ruggirello, A., Silvestri, A., Terenzi, A., & Turco, V. (2009). Spectroscopic study of the interaction of Ni II -5-triethyl ammonium methyl salicylidene ortho-phenylendiiminate with native DNA. Journal of Inorganic Biochemistry, 103, 731–737. https://doi.org/10.1016/j.jinorgbio.2009.01.006.
Moro, A. C., Urbaczek, A. C., De Almeida, E.T., Fernando, R., Leite, C. Q. F., Netto, A. V. G., Mauro, A. E., Moro, A. C., Urbaczek, A. C., De Almeida, E.T., Pavan, R., Leite, C. Q. F., Netto, A. V. G., Binuclear, Binuclear cyclopalladated compounds with antitubercular activity: synthesis and characterization of [{ Pd (C, N-dmba)(X)} 2 (µ -bpp)] (X = Cl, Br, NCO, N 3; bpp = 1, 3-bis (4-pyridyl) propane), 8972 (2012). https://doi.org/10.1080/00958972.2012.673718.
Mascaliovas, B. Z., Bergamini F. R. G., Cuin A., Corbi P. P., Synthesis and crystal structure of a palladium (II) complex with the amino acid L-citrulline, 30 (2015) 7156. https://doi.org/10.1017/S0885715615000652.
Patel, M. N., Dosi, P. A., Bhatt B. S., Square planar palladium (II) complexes of bipyridines: synthesis, characterization, and biological studies, 8972 (2012). https://doi.org/10.1080/00958972.2012.727207.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3.
Nakamura, Y., Gindhart, T. D., Winterstein, D., Tomita, I., Seed, J. L., & Colburn, N. H. (1988). Early superoxide dismutase-sensitive event promotes neoplastic transformation in mouse epidermal JB6 cells. Carcinogenesis., 9, 203–207. https://doi.org/10.1093/carcin/9.2.203.
Beers, R. F., & Sizer, I. W. (1951). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry, 24, 113–140.
Wendel, A. (1981). Glutathione peroxidase. Methods Enzymol, 77, 325–333. https://doi.org/10.1016/S0076-6879(81)77046-0.
Moron, M., Depierre, J., & Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta, 582, 67–78. https://doi.org/10.1016/0304-4165(79)90289-7.
Altay, A., Tohma, H., Durmaz, L., Taslimi, P., Korkmaz, M., Gulcin, I., & Koksal, E. (2019). Preliminary phytochemical analysis and evaluation of in vitro antioxidant, antiproliferative, antidiabetic, and anticholinergics effects of endemic Gypsophila taxa from Turkey. Journal of Food Biochemistry, 43, 1–11. https://doi.org/10.1111/jfbc.12908.
Banti, C. N. & Hadjikakou, S. K. (2016). Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in metal complexes and their effect at the cellular level. European Journal of Inorganic Chemistry, 3048–3071. https://doi.org/10.1002/ejic.201501480.
Totta, X., Papadopoulou, A. A., Hatzidimitriou, A. G., Papadopoulos, A., & Psomas, G. (2015). Synthesis, structure and biological activity of nickel (II) complexes with mefenamato and nitrogen-donor ligands. Journal of Inorganic Biochemistry, 145, 79–93. https://doi.org/10.1016/j.jinorgbio.2015.01.009.
Giovagnini, L., Marzano, C., Bettio, F., & Fregona, D. (2005). Mixed complexes of Pt(II) and Pd(II) with ethylsarcosinedithiocarbamate and 2-/3-picoline as antitumor agents. Journal of Inorganic Biochemistry, 99, 2139–2150. https://doi.org/10.1016/j.jinorgbio.2005.07.016.
Altay, A., Caglar, S., Caglar, B., & Sahin, Z. S. (2019). Novel silver(I) complexes bearing mefenamic acid and pyridine derivatives: synthesis, chemical characterization and in vitro anticancer evaluation. Inorganica Chimica Acta, 493, 61–71. https://doi.org/10.1016/j.ica.2019.05.008.
Altay, A., Caglar, S., Caglar, B., & Sahin, O. (2018). Synthesis, structural, thermal elucidation and in vitro anticancer activity of novel silver(I) complexes with non-steroidal anti-inflammatory drugs diclofenac and mefenamic acid including picoline derivatives. Polyhedron., 151, 160–170. https://doi.org/10.1016/j.poly.2018.05.038.
Gao, X., Li, X., Ho, C. T., Lin, X., Zhang, Y., Li, B., & Chen, Z. (2020). Cocoa tea (Camellia ptilophylla) induces mitochondria-dependent apoptosis in HCT116 cells via ROS generation and PI3K/Akt signaling pathway. Food Res. Int., 129, 108854. https://doi.org/10.1016/j.foodres.2019.108854.
Icsel, C., Yilmaz, V. T., Cevatemre, B., Aygun, M., & Ulukaya, E. (2019). Structures and anticancer activity of chlorido platinum(II) saccharinate complexes with mono- and dialkylphenylphosphines. Journal of Inorganic Biochemistry, 195, 39–50. https://doi.org/10.1016/j.jinorgbio.2019.03.008.
Wang, C., & Youle, R. J. (2009). The role of mitochondria in apoptosis. Annu. Rev. Genet., 43, 95–118. https://doi.org/10.1146/annurev-genet-102108-134850.
Yang, M., Wang, B., Gao, J., Zhang, Y., Xu, W., & Tao, L. (2017). Chemosphere Spinosad induces programmed cell death involves mitochondrial dysfunction and cytochrome C release in Spodoptera frugiperda Sf9 cells. Chemosphere, 169, 155–161. https://doi.org/10.1016/j.chemosphere.2016.11.065.
Wang, Y., Gao, W., Shi, X., Ding, J., Liu, W., He, H., Wang, K., & Shao, F. (2017). Caspase-3 cleavage of a gasdermin. Nature, 547, 99–103. https://doi.org/10.1038/nature22393.
Wang, Y., Liu, J., Qiu, Y., Jin, M., Chen, X., Fan, G., Wang, R., & Kong, D. (2016). ZSTK474, a specific class I phosphatidylinositol 3-kinase inhibitor, induces G1 arrest and autophagy in human breast cancer MCF-7 cells. Oncotarget., 7, 19897–19909. https://doi.org/10.18632/oncotarget.7658.
Lin, J., Adam, R. M., Santiestevan, E., & Freeman, M. R. (1999). The Phosphatidylinositol 3′-kinase Pathway Is a Dominant Growth Factor-activated Cell Survival Pathway in LNCaP Human Prostate Carcinoma Cells. Cancer Research, 59, 2891–2897.
Fry, M. J.(2001). Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Research, 3, 304–312.
Lin, X., Schulz, A., Schulz, A., Overexpression of phosphatidylinositol 3-kinase in human lung cancer, (2001) 293–301. https://doi.org/10.1007/s004230100203.
Nicholson, K. M., & Anderson, N. G. (2002). The protein kinase B/Akt signalling pathway in human malignancy. Cellular Signalling, 14, 381–395. https://doi.org/10.1016/S0898-6568(01)00271-6.
Lin, K., Rong, Y., Chen, D., Zhao, Z., Bo, H., Qiao, A., Hao, X., & Wang, J. (2020). Combination of ruthenium complex and doxorubicin synergistically inhibits cancer cell growth by down-regulating PI3K/AKT signaling pathway. Frontiers in Oncology, 10, 1–13. https://doi.org/10.3389/fonc.2020.00141.
Hua, Z., Dan, L., Qiao, W., Yi, Y., Yao, W., Yun, Z., & Liu, J. (2018). A cyclometalated iridium (III) complex induces apoptosis and autophagy through inhibition of the PI3K / AKT / mTOR pathway. Transition Metal Chemistry, 43, 243–257. https://doi.org/10.1007/s11243-018-0210-z.
Patel, P., Nadar, V. M., Umapathy, D., Manivannan, S., Venkatesan, R., Antony, V., Arokiyam, J., Pappu, S., Prakash, P. A., Padmanabhan, P., Doxorubicin-conjugated platinum theranostic nanoparticles induce apoptosis via inhibition of a cell survival (PI3K / AKT) signaling pathway in human breast cancer cells, (2020). https://doi.org/10.1021/acsanm.0c02521.
Bhattacharyya, A., Chattopadhyay, R., Mitra, S., Crowe, S. E., Bhattacharyya, A., Chattopadhyay, R., Mitra, S., Crowe, S. E., Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases, (2021) 329–354. https://doi.org/10.1152/physrev.00040.2012.
Zhang, P. & Sadler, P. J. (2017). Redox-active metal complexes for anticancer therapy. European Journal of Inorganic Chemistry, 1541–1548. https://doi.org/10.1002/ejic.201600908.
Barilli, A., Atzeri, C., Bassanetti, I., Ingoglia, F., Asta, V. D., Bussolati, O., Ma, M., Mucchino, C., & Marchio, L. (2014). Oxidative stress induced bycopper and iron complexes with 8‑hydroxyquinoline derivatives causes paraptotic death of HeLa cancer cells. American Chemical Society, 11, 1151–1163. https://doi.org/10.1021/mp400592n.
Mos, M., Sadler, P. J., Enhancement of selectivity of an organometallic anticancer agent by redox modulation, (2015). https://doi.org/10.1021/acs.jmedchem.5b00655.
Sadler, P. J.(2013). Next-generation metal anticancer complexes: multitargeting via redox modulation. American Chemical Society, 52, 12276–12291. https://doi.org/10.1021/ic400835n.
Al-Khayal, K., Vaali-Mohammed, M. A., Elwatidy, M., Bin Traiki, T., Al-Obeed, O., Azam, M., Khan, Z., Abdulla, M., & Ahmad, R. (2020). A novel coordination complex of platinum (PT) induces cell death in colorectal cancer by altering redox balance and modulating MAPK pathway. BMC Cancer., 20, 1–17. https://doi.org/10.1186/s12885-020-07165-w.
Guo, D., Xu, S., Huang, Y., Jiang, H., Yasen, W., Wang, N., Su, Y., Qian, J., Li, J., Zhang, C., & Zhu, X. (2018). Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials., 177, 67–77. https://doi.org/10.1016/j.biomaterials.2018.05.052.
Hsin, Y. H., Chen, C. F., Huang, S., Shih, T. S., Lai, P. S., & Chueh, P. J. (2008). The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicology Letters, 179, 130–139. https://doi.org/10.1016/j.toxlet.2008.04.015.
Grimm, E. A., Ellerhorst, J., Tang, C. H., & Ekmekcioglu, S. (2008). Constitutive intracellular production of iNOS and NO in human melanoma: possible role in regulation of growth and resistance to apoptosis. Nitric Oxide, 19, 133–137. https://doi.org/10.1016/j.niox.2008.04.009.
Korde Choudhari, S., Chaudhary, M., Bagde, S., Gadbail, A. R., & Joshi, V. (2013). Nitric oxide and cancer: a review. World Journal of Surgical Oncology, 11, 1–11. https://doi.org/10.1186/1477-7819-11-118.
Kim, P. K. M., Zamora, R., Petrosko, P., & Billiar, T. R. (2001). The regulatory role of nitric oxide in apoptosis. International Immunopharmacology, 1, 1421–1441. https://doi.org/10.1016/S1567-5769(01)00088-1.
Kohan, R., Collin, A., Guizzardi, S., Tolosa de Talamoni, N., & Picotto, G. (2020). Reactive oxygen species in cancer: a paradox between pro- and anti-tumour activities. Cancer Chemotherapy and Pharmacology, 86, 1–13. https://doi.org/10.1007/s00280-020-04103-2.
Güller, P., Budak, H., Şişecioğlu, M., & Çiftci, M. (2020). An in vivo and in vitro comparison of the effects of amoxicillin, gentamicin, and cefazolin sodium antibiotics on the mouse hepatic and renal glutathione reductase enzyme. Journal of Biochemical Molecular Toxicology, 34, 1–10. https://doi.org/10.1002/jbt.22496.
Altay, A., & Bozoğlu, F. (2017). Salvia fruticosa modulates mRNA expressions and activity levels of xenobiotic metabolizing CYP1A2, CYP2E1, NQO1, GPx, and GST enzymes in human colorectal adenocarcinoma HT-29 cells. Nutrition and Cancer, 69, 892–903. https://doi.org/10.1080/01635581.2017.1339817.
Devereux, M., Shea, D. O., Connor, M. O., Grehan, H., Connor, G., Mccann, M., Rosair, G., Lyng, F., Kellett, A., Walsh, M., Egan, D., & Thati, B. (2007). Synthesis, catalase, superoxide dismutase and antitumour activities of copper (II) carboxylate complexes incorporating benzimidazole 1, 10-phenanthroline and bipyridine ligands:. X-ray Cystal Structures, 26, 4073–4084. https://doi.org/10.1016/j.poly.2007.05.006.
Wert, K. J., Velez, G., Cross, M. R., Wagner, B. A., Teoh-fitzgerald, M. L., Buettner, G. R., Mcanany, J. J., Olivier, A., Tsang, S. H., Harper, M. M., Domann, F. E., & Bassuk, A. G. (2018). Extracellular superoxide dismutase (SOD3) regulates oxidative stress at the vitreoretinal interface ☆. Free Radical Biology and Medicine, 124, 408–419. https://doi.org/10.1016/j.freeradbiomed.2018.06.024.
Illán-cabeza, N. A., García-garcía, A. R., Martínez-martos, J. M., Ramírez-expósito, M. J., Peña-ruiz, T., & Moreno-carretero, M. N. (2013). Original article A potential antitumor agent, (6-amino-1-methyl-5-nitrosouracilato- N3) -triphenylphosphine-gold (I): structural studies and in vivo biological effects against experimental glioma. European Journal of Medicinal Chemistry, 64, 260–272. https://doi.org/10.1016/j.ejmech.2013.03.067.
Kovala-demertzi, D., Staninska, M., Garcia-santos, I., Castineiras, A., & Demertzis, M. A. (2011). Synthesis, crystal structures and spectroscopy of meclofenamic acid and its metal complexes with manganese (II), copper (II), zinc (II) and cadmium (II). Antiproliferative and superoxide dismutase activity. Journal of Inorganic Biochemistry, 105, 1187–1195. https://doi.org/10.1016/j.jinorgbio.2011.05.025.
Shahraki, S., Saeidifar, M., & Samareh, H. (2020). Molecular docking and inhibitory effects of a novel cytotoxic agent with bovine liver catalase. Journal of Molecular Structure, 1205, 127590. https://doi.org/10.1016/j.molstruc.2019.127590.
Signorella, S., Palopoli, C., & Ledesma, G. (2018). Rationally designed mimics of antioxidant manganoenzymes: role of structural features in the quest for catalysts with catalase and superoxide dismutase activity. Coordination Chemistry Reviews, 365, 75–102. https://doi.org/10.1016/j.ccr.2018.03.005.
Altay, A., Kartal, D. I., Sadi, G., Güray, T., & Yaprak, A. E. (2017). Modulation of mRNA expression and activities of xenobiotic metabolizing enzymes, CYP1A1, CYP1A2, CYP2E1, GPx and GSTP1 by the Salicornia freitagii extract in HT-29 human colon cancer cells. Archives of Biological Sciences, 69, 439–448. https://doi.org/10.2298/ABS160825118A.
Ceylan, H., Budak, H., Fehim, E., & Gonul, N. (2019). Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. Journal of Trace Elements in Medicine and Biology, 56, 198–206. https://doi.org/10.1016/j.jtemb.2019.09.002.
Franco, J. L., Posser, T., Dunkley, P. R., Dickson, P. W., Mattos, J. J., Martins, R., Bainy, A. C. D., Marques, M. R., Dafre, A. L., & Farina, M. (2009). Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radical Biology & Medicine, 47, 449–457. https://doi.org/10.1016/j.freeradbiomed.2009.05.013.
Gross, A. (2016). BCL-2 family proteins as regulators of mitochondria metabolism ☆. Biochimica et Biophysica Acta, 1857, 1243–1246. https://doi.org/10.1016/j.bbabio.2016.01.017.
Zhang, J., Huang, K., Neill, K. L. O., Pang, X., Luo, X., Bax/Bak activation in the absence of Bid, Bim, Puma, and p53, Nature Publishing Group (2016). https://doi.org/10.1038/cddis.2016.167.
Acknowledgements
This work was financially supported by grants from Erzincan University Scientific Research Projects Coordination Commission (EU-BAP) (Project No: FBA-2016-381).
Author Contributions
A.A., S.C. and M.K. M.K. performed the experiments. A.A. and B.C. designed and analyzed the experiments. A.A. and S.C. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Caglar, S., Altay, A., Kuzucu, M. et al. In Vitro Anticancer Activity of Novel Co(II) and Ni(II) Complexes of Non-steroidal Anti-inflammatory Drug Niflumic Acid Against Human Breast Adenocarcinoma MCF-7 Cells. Cell Biochem Biophys 79, 729–746 (2021). https://doi.org/10.1007/s12013-021-00984-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-00984-z