Abstract
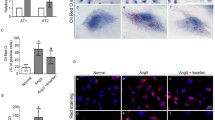
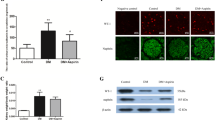
Previous studies have shown that lipid accumulation plays an important role in the pathogenesis and development of glomerular sclerosis. oxLDL caused damage in renal mesangial cells, endothelial cells, and podocytes, and podocytes might be the major victim of oxLDL insult. However, the regulatory mechanism of how oxLDL induces the damage of podocytes remains to be elucidated. In this study, oil red staining was used to investigate the lipid accumulation in podocytes. Moreover, the effects of CXCL16 antibody, IFN-γ, and ADAM10 inhibitor on oxLDL intake and CXCL16 expression were also explored to elucidate the regulatory factors of lipid accumulation in podocytes.









Similar content being viewed by others
References
Moorhead, J. F., et al. (1982). Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet, 2(8311), 1309–1311.
Moorhead, J. F., Brunton, C., & Varghese, Z. (1997). Glomerular atherosclerosis. Mineral and Electrolyte Metabolism, 23(3–6), 287–290.
Xin Wang, D. S. (2008). Progress in the mechanism of hyperlipidemia in glomerular injury. Journal of Practical Medicine, 24(7), 2.
Joles, J. A., et al. (2000). Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. Journal of the American Society of Nephrology, 11(4), 669–683.
Wiley, A., et al. (2006). Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer, 107(2), 299–308.
Weiguo Li, Y. L. (2012). Podocytes response to injury. Journal of Clinical Pediatrics, 30(11), 4.
Bussolati, B., et al. (2005). Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. Journal of the American Society of Nephrology, 16(7), 1936–1947.
Gutwein, P., et al. (2009). CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. American Journal of Pathology, 174(6), 2061–2072.
Mundel, P., & Shankland, S. J. (2002). Podocyte biology and response to injury. Journal of the American Society of Nephrology, 13(12), 3005–3015.
Nosadini, R., & Tonolo, G. (2011). Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutrition, Metabolism & Cardiovascular Diseases, 21(2), 79–85.
Stitt-Cavanagh, E., MacLeod, L., & Kennedy, C. (2009). The podocyte in diabetic kidney disease. Scientific World Journal, 9, 1127–1139.
Zheng, Q. Z. X. (2011). Advances in understanding of podocyte function. Medical Review, 17(19), 4.
Gutwein, P., et al. (2009). CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. Journal of Cellular and Molecular Medicine, 13(9B), 3809–3825.
Wiggins, R. C. (2007). The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney International, 71(12), 1205–1214.
Mundel, P., et al. (1997). Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Experimental Cell Research, 236(1), 248–258.
Yang, D. L. H. (2012). Oxidized low-density lipoprotein and glomerular sclerosis. China in Integrative Medicine, 13(12), 2.
Wang, H. C. Z. T. Y. (2006). Lipid effects of mouse kidney podocyte proliferation. Journal of Medical Research, 35(8), 4.
Hu, Y., & Zhihua Quan, Y. W. (2010). Chemokine CXCL16 New Progress. Medical Review, 16(8), 4.
Ruixia Xu, J. L. (2012). Oxidized low-density lipoprotein cholesterol and atherosclerosis. Chinese Circulation Journal, 27(3), 5.
Qing Lin, Y. S., & Zhu, X. (2013). IFN-γ-induced tubular epithelial cells CXCL9, CXCL10 and CXCL11 expression. Journal of Cellular & Molecular Immunology, 29(2), 4.
Wuttge, D. M., et al. (2004). CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(4), 750–755.
Ting Yang, Z. Q. (2008). Interferon smooth muscle cells of the murine CXC Ligand 16 Expression and intracellular lipid accumulation effects. Chinese Journal of Arteriosclerosis, 16(3), 4.
Leon, M. L., & Zuckerman, S. H. (2005). Gamma interferon: a central mediator in atherosclerosis. Inflammation Research, 54(10), 395–411.
Gough, P. J., et al. (2004). A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. Journal of Immunology, 172(6), 3678–3685.
Ludwig, A., et al. (2005). Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Combinatorial Chemistry & High Throughput Screening, 8(2), 161–171.
Abel, S., et al. (2004). The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. Journal of Immunology, 172(10), 6362–6372.
Shimaoka, T., et al. (2004). Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. Journal of Leukocyte Biology, 75(2), 267–274.
Acknowledgments
This study was supported by Shandong Province Natural Science Foundation (No. ZR2010HM110).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Sun, S., Zhou, A. et al. oxLDL-Induced Lipid Accumulation in Glomerular Podocytes: Role of IFN-γ, CXCL16, and ADAM10. Cell Biochem Biophys 70, 529–538 (2014). https://doi.org/10.1007/s12013-014-9952-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9952-1