Abstract
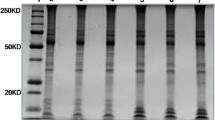
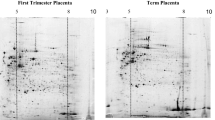
To explore the proteomic changes of placental trophoblastic cells in preeclampsia–eclampsia (PE), placental trophoblastic cells from normally pregnant women and women with hypertension during gestational period were prepared by laser capture microdissection (LCM), and proteins isolated from these cells were subjected to labeling and proteolysis with isotope-coded affinity tag reagent. A qualitative and quantitative analysis of the proteome expression of placental trophoblastic cells was made using two-dimensional liquid chromatography tandem mass spectrometry (2D LC–MS/MS). A total of 831 proteins in placental trophoblastic cells were identified by combined use of LCM technique and 2D LC–MS/MS. The result was superior to that of conventional two-dimensional electrophoresis method. There were marked differences in 169 proteins of placental trophoblastic cells between normally pregnant women and women with PE. Of 70 (41.4 %) proteins with more than twofold differences, 31 proteins were down-regulated, and 39 were up-regulated in placental trophoblastic cells of the woman with PE. Laminin expression in placenta trophoblastic cells of women with PE was significantly down-regulated as confirmed by Western blot analysis. These findings provide insights into the proteomic changes in placental trophoblastic cells in response to PE and may identify novel protein targets associated with the pathogenesis of PE.






Similar content being viewed by others
References
Cunningham, F. G., Gant, N. F., Leveno, K. J., et al. (2001). Hypertensive disorders of pregnancy. In F. G. Cunningham, N. F. Gant, K. J. Leveno, et al. (Eds.), Williams obstetrics (21st ed.). New York: McGraw-Hill.
Speake, P. F., Glazier, J. D., Ayuk, P. T., et al. (2003). l-Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. The Journal of clinical endocrinology and metabolism, 88, 4287–4292.
Vedernikov, Y., Saade, G. R., & Garfield, R. E. (1999). Vascular reactivity in preeclampsia. Seminars in Perinatology, 23, 34–44.
Goldman-Wohl, D., & Yagel, S. (2002). Regulation of trophoblast invasion: From normal implantation to pre-eclampsia. Molecular and Cellular Endocrinology, 187, 233–238.
Hirano, H., Imai, Y., & Ito, H. (2002). Spiral artery of placenta: Development and pathology-immunohistochemical, microscopical, and electron-microscopic study. The Kobe journal of medical sciences, 48, 13–23.
Robillard, P. Y. (2002). Interest in preeclampsia for researchers in reproduction. Journal of Reproductive Immunology, 2002(53), 279–287.
Cartwright, J. E., Tse, W. K., & Whitley, G. S. (2002). Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Experimental Cell Research, 279, 219–226.
Grosfeld, A., Turban, S., Andre, J., et al. (2001). Transcriptional effect of hypoxia on placental leptin. FEBS Letters, 502, 122–126.
Gratton, R. J., Asano, H., & Han, V. K. (2002). The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with pre-eclampsia. Placenta, 23, 303–310.
Shaarawy, M., El Meleigy, M., & Rasheed, K. (2001). Maternal serum transforming growth factor beta-2 in preeclampsia and eclampsia, a potential biomarker for the assessment of disease severity and fetal outcome. Journal of the Society for Gynecologic Investigation, 8, 27–31.
O’Brien, M., Dausset, J., Carosella, E. D., et al. (2000). Analysis of the role of HLA-G in preeclampsia. Human Immunology, 61, 1126–1131.
Xia, Y., Wen, H., Bobst, S., et al. (2003). Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. Journal of the Society for Gynecologic Investigation, 10, 82–93.
Allaire, A. D., Ballenger, K. A., Wells, S. R., et al. (2000). Placental apoptosis in preeclampsia. Obstetrics and Gynecology, 96, 271–276.
Reister, F., Frank, H. G., Kingdom, J. C., et al. (2001). Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Laboratory Investigation: A Journal of Technical Methods and Pathology, 81, 1143–1152.
Ishihara, N., Matsuo, H., Murakoshi, H., et al. (2002). Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. American Journal of Obstetrics and Gynecology, 186, 158–166.
Arechavaleta-Velasco, F., Koi, H., Strauss, J. F, 3rd, et al. (2002). Viral infection of the trophoblast: Time to take a serious look at its role in abnormal implantation and placentation? Journal of Reproductive Immunology, 55, 113–121.
Kanayama, N. (2003). Trophoblastic injury: New etiological and pathological concept of preeclampsia. Croatian Medical Journal, 44, 148–156.
Hoang, V. M., Foulk, R., Clauser, K., et al. (2001). Functional proteomics: Examining the effects of hypoxia on the cytotrophoblast protein repertoire. Biochemistry, 40, 4077–4086.
Hu, R., Jin, H., Zhou, S., et al. (2007). Proteomic analysis of hypoxia-induced responses in the syncytialization of human placental cell line BeWo. Placenta, 28, 399–407.
Sun, L. Z., Yang, N. N., De, W., et al. (2007). Proteomic analysis of proteins differentially expressed in preeclamptic trophoblasts. Gynecologic and Obstetric Investigation, 64, 17–23.
Mine, K., Katayama, A., Matsumura, T., et al. (2007). Proteome analysis of human placentae: Pre-eclampsia versus normal pregnancy. Placenta, 28, 676–687.
Shankar, R., Cullinane, F., Brennecke, S. P., et al. (2004). Applications of proteomic methodologies to human pregnancy research: A growing gestation approaching delivery? Proteomics, 4, 1909–1917.
Craven, R. A., Totty, N., Harnden, P., et al. (2002). Laser capture microdissection and two-dimensional polyacrylamide gel electrophoresis: Evaluation of tissue preparation and sample limitations. The American Journal of Pathology., 160, 815–822.
Li, C., Hong, Y., Tan, Y. X., et al. (2004). Accurate qualitative and quantitative proteomic analysis of clinical hepatocellular carcinoma using laser capture microdissection coupled with isotope-coded affinity tag and two-dimensional liquid chromatography mass spectrometry. Molecular & cellular proteomics: MCP., 3, 399–409.
Emmert-Buck, M. R., Bonner, R. F., Smith, P. D., et al. (1996). Laser capture microdissection. Science, 274, 998–1001.
Bonner, R. F., Emmert-Buck, M., Cole, K., et al. (1997). Laser capture microdissection: Molecular analysis of tissue. Science, 278(1481), 1483.
Goldsworthy, S. M., Stockton, P. S., Trempus, C. S., et al. (1999). Effects of fixation on RNA extraction and amplification from laser capture microdissected tissue. Molecular Carcinogenesis, 25, 86–91.
Kitahara, O., Furukawa, Y., Tanaka, T., et al. (2001). Alterations of gene expression during colorectal carcinogenesis revealed by cDNA microarrays after laser-capture microdissection of tumor tissues and normal epithelia. Cancer Research, 61, 3544–3549.
Wittliff, J. L., & Erlander, M. G. (2002). Laser capture microdissection and its applications in genomics and proteomics. Methods in Enzymology, 356, 12–25.
Gygi, S. P., Rist, B., Gerber, S. A., et al. (1999). Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature Biotechnology, 17, 994–999.
Goshe, M. B., Blonder, J., & Smith, R. D. (2003). Affinity labeling of highly hydrophobic integral membrane proteins for proteome-wide analysis. Journal of Proteome Research, 2, 153–161.
Kubota, K., Wakabayashi, K., & Matsuoka, T. (2003). Proteome analysis of secreted proteins during osteoclast differentiation using two different methods: Two-dimensional electrophoresis and isotope-coded affinity tags analysis with two-dimensional chromatography. Proteomics, 3, 616–626.
Ranish, J. A., Yi, E. C., Leslie, D. M., et al. (2003). The study of macromolecular complexes by quantitative proteomics. Nature Genetics, 33, 349–355.
Jin, J., Meredith, G. E., Chen, L., et al. (2005). Quantitative proteomic analysis of mitochondrial proteins: Relevance to Lewy body formation and Parkinson’s disease. Brain Research. Molecular Brain Research, 134, 119–138.
Wang, Y., Rudnick, P. A., Evans, E. L., et al. (2005). Proteome analysis of microdissected tumor tissue using a capillary isoelectric focusing-based multidimensional separation platform coupled with ESI-tandem MS. Analytical Chemistry, 77, 6549–6556.
Zhang, J., Goodlett, D. R., Quinn, J. F., et al. (2005). Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer disease. Journal of Alzheimer’s disease: JAD., 7, 125–133. discussion 173–180.
Schmidt, F., Donahoe, S., Hagens, K., et al. (2004). Complementary analysis of the Mycobacterium tuberculosis proteome by two-dimensional electrophoresis and isotope-coded affinity tag technology. Molecular & cellular proteomics: MCP., 3, 24–42.
Smolka, M. B., Zhou, H., Purkayastha, S., et al. (2001). Optimization of the isotope-coded affinity tag-labeling procedure for quantitative proteome analysis. Analytical Biochemistry, 297, 25–31.
Gygi, S. P., Corthals, G. L., Zhang, Y., et al. (2000). Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proceedings of the National Academy of Sciences of the United States of America, 97, 9390–9395.
Washburn, M. P., Wolters, D., & Yates, J. R, 3rd. (2001). Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nature Biotechnology, 19, 242–247.
Korhonen, M., & Virtanen, I. (1997). The distribution of laminins and fibronectins is modulated during extravillous trophoblastic cell differentiation and decidual cell response to invasion in the human placenta. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society., 45, 569–581.
Pijnenborg, R., D’Hooghe, T., Vercruysse, L., et al. (1996). Evaluation of trophoblast invasion in placental bed biopsies of the baboon, with immunohistochemical localisation of cytokeratin, fibronectin, and laminin. Journal of Medical Primatology, 25, 272–281.
Loke, Y. W., Burrows, T., & King, A. (1995). Adhesion molecules and trophoblastic invasion. Contraception, Fertilite, Sexualite, 23, 573–575.
Laurie, G. W., Bing, J. T., Kleinman, H. K., et al. (1986). Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. Journal of Molecular Biology, 189, 205–216.
Sasaki, M., Kato, S., Kohno, K., et al. (1987). Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proceedings of the National Academy of Sciences of the United States of America, 84, 935–939.
Turpeenniemi-Hujanen, T., Ronnberg, L., Kauppila, A., et al. (1992). Laminin in the human embryo implantation: Analogy to the invasion by malignant cells. Fertility and Sterility, 58, 105–113.
Korhonen, M., & Virtanen, I. (2001). Immunohistochemical localization of laminin and fibronectin isoforms in human placental villi. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society., 49, 313–322.
Dekker, G. A., & Sibai, B. M. (1998). Etiology and pathogenesis of preeclampsia: Current concepts. American Journal of Obstetrics and Gynecology, 179, 1359–1375.
van Beck, E., & Peeters, L. L. (1998). Pathogenesis of preeclampsia: A comprehensive model. Obstetrical & Gynecological Survey, 53, 233–239.
Sanjuan, X., Fernandez, P. L., Miquel, R., et al. (1996). Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. The Journal of Pathology, 179, 376–380.
Risteli, J., Foidart, J. M., Risteli, L., et al. (1984). The basement membrane proteins laminin and type IV collagen in isolated villi in pre-eclampsia. Placenta, 5, 541–550.
Acknowledgments
This research was supported by the National Nature Science Foundation of China (Grant No. 3060075) and the Foundation of China postdoctoral Science (Grant No. 20060400147) and the Foundation of Shanghai postdoctoral, China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Kaidong Ma and Hong Jin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ma, K., Jin, H., Hu, R. et al. A Proteomic Analysis of Placental Trophoblastic Cells in Preeclampsia–Eclampsia. Cell Biochem Biophys 69, 247–258 (2014). https://doi.org/10.1007/s12013-013-9792-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9792-4