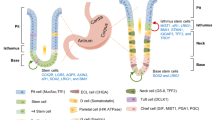
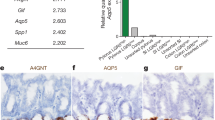
Abstract
Gastric cancer is one of the most outgoing human cancers in the world. Two main functional types were described: Intestinal adenocarcinoma and diffuse one. The most important purpose of this review is to analyze and investigate the main genetic factors involved in tumorogenesis of stomach and the molecular mechanism of their expression regulation alongside with the importance of cancer stem cells and their relationship with gastric cancer. It is evident that proper diagnosis of molecular case of cancer may lead to absolute treatment and at least reduction in the disease severity. However, stemness factors such as Sox2, Oct3/4, and Nanog were related with induced pluripotent stem cells, proposing a correlation between these stemness factors and cancer stem cells. Moreover, aberrant induction by Helicobacter pylori of the intestinal-specific homeobox transcription factors, CDX1 and CDX2, also plays an important role in this modification. There are some genes which are directly activated by CDX1 in gastric cancer and distinguished stemness-related reprogramming factors like SALL4 and KLF5. Correspondingly, we also aimed to present the main important epigenetic changes such as DNA methylation, histone modification, and chromatin modeling of stemness genes in disease development. Remarkably, a better understanding of molecular bases of cancer may lead to novel diagnostic, therapeutic, and preventive approaches by some genetic and epigenetic changes such as gene amplifications, gene silencing by DNA methylation, losses of imprinting, LOH, and mutations. Consequently, genome-wide searches of gene expression are widely important for surveying the proper mechanisms of cancer emergence and development. Conspicuously, this review explains an outline of the molecular mechanism and new approaches in gastric cancer.



Similar content being viewed by others
References
Vaccaro, B. J., Gonzalez, S., Poneros, J. M., Stevens, P. D., Capiak, K. M., Lightdale, C. J., et al. (2011). Detection of intestinal metaplasia after successful eradication of Barrett’s Esophagus with radiofrequency ablation. Digestive Diseases and Sciences, 56(7), 1996–2000.
Cunningham, S. C., Kamangar, F., Kim, M. P., Hammoud, S., Haque, R., Iacobuzio-Donahue, C. A., et al. (2006). Claudin-4, mitogen-activated protein kinase kinase 4, and stratifin are markers of gastric adenocarcinoma precursor lesions. Cancer Epidemiology Biomarkers and Prevention, 15, 281–287.
Yoshikawa, A., Inada, K. I., Yamachika, T., Shimizu, N., Kaminishi, M., & Tatematsu, M. (1998). Phenotypic shift in human differentiated gastric cancers from gastric to intestinal epithelial cell type during disease progression. Gastric Cancer, 1, 134–141.
Almeida, R., Silva, E., Santos-Silva, F., Silberg, D. G., Wang, J., De Bolos, C., et al. (2003). Expression of intestine-specific transcription factor, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. The Journal of Pathology, 199, 36–40.
Tsukamoto, T., Mizoshita, T., Mihara, M., Tanaka, H., Takenaka, Y., Yamamura, Y., et al. (2005). Sox2 expression in human stomach adenocarcinomas with gastric and gastric-and-intestinal-mixed phenotypes. Histopathology, 46, 649–658.
Meireles, S. I., Cristo, E. B., Carvalho, A. F., Hirata, R, Jr, Pelosof, A., Gomes, L. I., et al. (2004). Molecular classifiers for gastric cancer and nonmalignant diseases of the gastric mucosa. Cancer Research, 64, 1255–1265.
Crawford, J. (1994). The gastrointestinal tract. In S. L. C. R. Robbins, V. Kumar, & F. J. Schoen (Eds.), Pathologic basis of disease (pp. 755–783). Philadelphia: WB Saunders Co.
Schwartz, G. (1996). Invasion and metastasis in gastric cancer: In vitro and in vivo models with clinical considerations. Seminars in Oncology, 23, 316–324.
Green, D., Ponce de Leon, S., Leon-Rodriguez, E., et al. (2002). Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. American Journal of Oncology, 25, 84–89.
Gore, R. (1997). Gastrointestinal cancer. Radiologic Clinics of North America, 35, 295–310.
Karpeh, M., & Brennan, M. (1998). Gastric carcinoma. Annals of Surgical Oncology, 5, 650–656.
Hundahl, S. A., Phillips, J. L., & Menck, H. R. (2000). The National Cancer Data Base Report on poor survival of US gastric carcinoma patients treated with gastrectomy: Fifth edition American Joint Committee on Cancer staging, proximal disease, and the ‘different disease’ hypothesis. Cancer, 88, 921–932.
Milne, A. N., Carneiro, F., O’Morain, C., & Offerhaus, G. J. (2009). Nature meets nurture: Molecular genetics of gastric cancer. Human Genetics, 126, 615–628.
Sugimoto, M., Furuta, T., Shirai, N., Nakamura, A., Kajimura, M., et al. (2007). Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. Journal of Gastroenterology and Hepatology, 22, 1443–1449.
Moy, K. A., Fan, Y., Wang, R., Gao, Y. T., Yu, M. C., et al. (2010). Alcohol and tobacco use in relation to gastric cancer: A prospective study of men in Shanghai, China. Cancer Epidemiology Biomarkers and Prevention, 19, 2287–2297.
Griffiths, E. A., Pritchard, S. A., Valentine, H. R., Whitchelo, N., Bishop, P. W., Ebert, M. P., et al. (2007). Hypoxia-inducible factor-1alpha expression in the gastric carcinogenesis sequence and its prognostic role in gastric and gastro-oesophageal adenocarcinomas. British Journal of Cancer, 96, 95–103.
Gendron, F. P., Mongrain, S., Laprise, P., McMahon, S., Dubois, C. M., Blais, M., et al. (2006). The CDX2 transcription factor regulates furin expression during intestinal epithelial cell differentiation. American Journal of Physiology. Gastrointestinal and Liver Physiology, 290, G310–G318.
Guo, R. J., Suh, E. R., & Lynch, J. P. (2004). The role of Cdx proteins in intestinal development and cancer. Cancer Biology & Therapy, 3, 593–601.
Silberg, D. G., Sullivan, J., Kang, E., Swain, G. P., Moffett, J., Sund, N. J., et al. (2002). Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology, 122, 689–696.
Mesquita, P., Jonckheere, N., Almeida, R., Ducourouble, M. P., Serpa, J., Silva, E., et al. (2003). Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. Journal of Biological Chemistry, 278, 51549–51556.
Tamura, G. (2006). Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World Journal of Gastroenterology, 12, 192–198.
Akhavan Niaki, H., & Samadani, A. A. (2013). DNA methylation and cancer development: Molecular mechanism. Cell Biochemistry and Biophysics. doi:10.1007/s12013-013-9555-2.
Sugai, T., Habano, W., Uesugi, N., Jao, Y. F., Nakamura, S., Abe, K., et al. (2004). Three independent genetic profiles based on mucin expression in early differentiated-type gastric cancer—A new concept of genetic carcinogenesis of early differentiated-type adenocarcinomas. Modern Pathology, 17, 1223–1234.
Teixeira, A., David, L., Reis, C. A., Costa, J., & Sobrinho-Simoes, M. (2002). Expression of mucins (MUC1, MUC2, MUC5AC, and MUC6) and type 1 lewis antigens in cases with and without Helicobacter pylori colonization in metaplastic glands of the human stomach. The Journal of Pathology, 197, 37–43.
Babu, S. D., Jayanthi, V., Devaraj, N., Reis, C. A., & Devaraj, H. (2006). Expression profile of mucins (MUC2, MUC5AC and MUC6) in Helicobacter pylori infected pre-neoplastic and neoplastic human gastric epithelium. Mol Cancer, 5, 10.
Johanson, A. H., Frierson, H. F., Zaika, A., Powell, S. M., Roche, J., Crowe, S., et al. (2005). Expression of tight-junction protein claudin-7 is an early event in gastric tumorigenesis. American Journal of Pathology, 167, 577–584.
Brenner, H., Arndt, V., Bode, G., Stegmaier, C., Ziegler, H., et al. (2002). Risk of gastric cancer among smokers infected with Helicobacter pylori. International Journal of Cancer, 98, 446–449.
Hatakeyama, M. (2004). Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nature Reviews Cancer, 4, 688–694.
Tsutsumi, R., Takahashi, A., Azuma, T., Higashi, H., & Hatakeyama, M. (2006). Focal adhesion kinase is a substrate and downstream effector of SHP-1 complexed with Helicobacter pylori CagA. Molecular and Cellular Biology, 26, 261–276.
Aung, P. P., Oue, N., Mitani, Y., Nakayama, H., Koshida, K., Noguchi, T., et al. (2006). Systematic search for gastric cancer-specific genes based on SAGE date: Melanoma inhibitory activity and matrix metalloproteinase-10 are novel prognostic factors in patients with gastric cancer. Oncogene, 25, 2546–2557.
Oue, N., Mitani, Y., Aung, P. P., Sakakura, C., Takeshima, Y., Kaneko, M., et al. (2005). Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. The Journal of Pathology, 207, 185–198.
Almeida, R., Almeida, J., Shoshkes, M., Mendes, N., Mesquita, P., Silva, E., et al. (2005). OCT- I is over-expression in intestinal metaplasia and intestinal gastric carcinomas and binds to, but does not transactivate, CDX2 in gastric cells. The Journal of Pathology, 207, 396–401.
Norbury, C., & Nurse, D. (1992). Animal cell cycles and their control. Review of Biochemistry, 61, 441–470.
Marx, J. (1994). How cells cycle toward cancer. Science (WashingtonDC), 263, 321–379.
Elledge, S. J., & Harper, J. W. (1996). A question of balance: The role of cyclin-kinase inhibitors in development and tumorigenesis. Trends in Cell Biology, 6, 388–392.
Hall, M., Bates, S., & Peter, G. (1995). Evidence for different mode of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinase, p21 and p27 bind to cyclins. Oncogene, 11, 1581–1588.
Chen, J., Kornbluth, P. S., Dynlacht, B. D., & Dutta, A. (1996). Cyclin-binding motifs are essential for the function of p21CIP1. Molecular and Cellular Biology, 16, 4673–4682.
Matsuoka, S., Edwards, M. C., Parker, S., Zhang, P., Baldini, A., Harper, J. W., et al. (1995). p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes & Development, 9, 650–662.
Polyak, K., Lee, M.-H., Erdjument-Bromage, Koff, A., Roberts, J. M., Tempst, P., et al. (1994). Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and potential mediator of extracellular antimitogenic signals. Cell, 78, 59–66.
Michieli, P., Chedid, M., Lin, D., Pierce, J. H., Mercer, W. E., & Givol, D. (1994). Induction of WAFI/CIPI by a p53-independent pathway. Cancer Research, 54, 3391–3395.
Margarita, S. B., Ana, I. S., Juan, C. M., Mateo, M. S., Lydia, S. V., Raquel, V., et al. (1997). Cyclin-dependent kinase inhibitor p27KIP1 in lymphoid tissue; P27 expression is inversely proportional to the proliferative index. American Journal of Pathology, 151, 151–159.
Silva, E., Teixeira, A., David, L., Carneiro, F., Reis, C. A., Sobrinho-Simoes, J., et al. (2002). Mucins as key molecules for the classification of intestinal metaplasia of the stomach. Virchows Archiv, 440, 311–317.
Archer, S. Y., & Hodin, R. A. (1999). Histone acetylation and cancer. Current Opinion in Genetics & Development, 9, 171–174.
Issa, J.-P. J., Ottaviano, Y. L., Celano, P., Hamilton, S. R., Davidson, N. E., & Baylin, S. B. (1994). Methylation of estrogen receptor CpG Island links aging and neoplasia in human colon. Nature Genetics, 7, 536–540.
Hamilton, S. R., & Aaltonen, L. A. (Eds.). (2000). Pathology and genetics of tumours of the digestive system. World Health Organization Classification of Tumours. Lyon: IARC Press.
Jones, P. A., & Taylor, S. M. (1980). Cellular differentiation. Cytidine analogs, and DNA methylation. Cell, 20, 85–93.
Weinstein, I. B. (2000). Disorders in cell circuitry during multistage carcinogenesis: The role of homeostasis. Carcinogenesis, 21(5), 857–864.
Greenman, C., et al. (2007). Patterns of somatic mutation in human cancer genomes. Nature, 446(7132), 153–158.
Vogelstein, B., & Kinzler, K. W. (2004). Cancer genes and the pathways they control. Nature Medicine, 10(8), 789–799.
Diehl, K. M., Keller, E. T., & Ignatoski, K. M. (2007). Why should we still care about oncogenes? Molecular Cancer Therapeutics, 6(2), 418–427.
Croce, C. M. (2008). Oncogenes and cancer. New England Journal of Medicine, 358(5), 502–511.
Schubbert, S., Shannon, K., & Bollag, G. (2007). Hyperactive Ras in developmental disorders and cancer. Nature Reviews Cancer, 7(4), 295–308.
Forbes, S. A., Bhamra, G., Bamford, S., Dawson, E., Kok, C., Clements, J., et al. (2008). The catalogue of somatic mutations in cancer (COSMIC). Current Protocols in Human Genetic, Chapter 10, Unit 10–11. doi:10.1002/0471142905.hg1011s57.
Kraft, P., Yen, Y. C., Stram, D. O., Morrison, J., & Gauderman, W. J. (2007). Exploiting gene–environment interaction to detect genetic associations. Human Heredity, 63, 111–119.
Jimeno, A., & Hidalgo, M. (2006). Molecular biomarkers: Their increasing role in the diagnosis, characterization, and therapy guidance in pancreatic cancer. Molecular Cancer Therapeutics, 5(4), 787–796.
Haug, U., Wente, M. N., Seiler, C. M., Jesenofsky, R., & Brenner, H. (2011). Stool testing for the early detection of pancreatic cancer: Rationale and current evidence. Expert Review of Molecular Diagnostics, 8(6), 753–759.
Kim, C. G., Choi, I. J., Lee, J. Y., Cho, S. J., Nam, B. H., et al. (2009). Biopsy site for detecting Helicobacter pylori infection in patients with gastric cancer. Journal of Gastroenterology and Hepatology, 24, 469–474.
van Krieken, J. H., et al. (2008). KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: Proposal for an European quality assurance program. Virchows Archiv, 453(5), 417–431.
Edkins, S., et al. (2006). Recurrent KRAS codon 146 mutations in human colorectal cancer. Cancer Biology & Therapy, 5(8), 928–932.
Brenner, H., Arndt, V., Bode, G., Stegmaier, C., Ziegler, H., et al. (2002). Risk of gastric cancer among smokers infected with Helicobacter pylori. International Journal of Cancer, 98, 446–449.
Shimoyama, T., Everett, S. M., Fukuda, S., Axon, A. T., Dixon, M. F., & Crabtree, J. E. (2001). Influence of smoking and alcohol on gastric chemokine mRNA expression in patients with Helicobacter pylori infection. Journal of Clinical Pathology, 54(4), 332–334.
Zambon, C. F., Basso, D., Navaglia, F., Belluco, C., Falda, A., et al. (2005). Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: Interactions influence outcome. Cytokine, 29, 141–152.
Ince, W. L., et al. (2005). Association of k-ras, and p53 status with the treatment effect of bevacizumab. Journal of the National Cancer Institute, 97(13), 981–989.
Wang, C., et al. (2003). Prognostic significance microsatellite instability and Ki-ras mutation type in stage II colorectal cancer. Oncology, 64(3), 259–265.
Ogino, S., et al. (2009). CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut, 58(1), 90–96.
Garcia-Gonzalez, M. A., Lanas, A., Quintero, E., Nicolas, D., Parra-Blanco, A., et al. (2007). Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: A Nationwide Multicenter Study in Spain. American Journal of Gastroenterology, 102, 1878–1892.
Filipowicz, W., Bhattacharyya, S. N., & Sonenberg, N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nature Reviews Genetics, 9, 102–114.
Papagiannakopoulos, T., & Kosik, K. S. (2008). MicroRNAs: Regulators of oncogenesis and stemness. BMC Med, 6, 15.
Wijnhoven, B. P., Michael, M. Z., & Watson, D. I. (2007). MicroRNAs and cancer. British Journal of Surgery, 94, 23–30.
Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116, 281–297.
Zhang, B., Pan, X., Cobb, G. P., & Anderson, T. A. (2007). MicroRNAs as oncogenes and tumor suppressors. Developmental Biology, 302, 1–12.
He, L., & Hannon, G. J. (2004). MicroRNAs: Small RNAs with a big role in gene regulation. Nature Reviews Genetics, 5, 522–531.
Allegra, E., & Trapasso, S. (2012). Cancer stem cells in head and neck cancer. Onco targets and therapy, 5, 375–383.
Prince, M. E., Sivanandan, R., Kaczorowski, A., et al. (2007). Identification of a subpopulation of with cancer stem cell properties in head and neck squamous cell carcinoma. Proceeding of the National Academy of Sciences USA, 104, 973–978.
Scadden, D. T. (2006). The stem-cell niche as an entity of action. Nature, 441, 1075–1079.
Li, L., & Xie, T. (2005). Stem cell niche: Structure and function. Annual Review of Cell and Developmental Biology, 21, 605–631.
Scadden, D. T. (2006). The stem-cell niche as an entity of action. Nature, 441, 1075–1079.
Yapeng, Hu, & Liwu, Fu. (2012). Targeting cancer stem cells. American Journal of Cancer Research, 2(3), 340–356.
La porta, C. A. M. (2012). Thoughts about cancer Stem cells in solid tumors. World Journal of Stem Cells, 4(3), 17–20.
Hellsten, R., Johansson, M., Dahlman, A., Sterner, O., & Biartell, A. (2011). Galiellalactone inhibits stem cell-like ALDH- positive prostate cancer cells. PLoS ONE, 6, e22118.
Dou, J., Jiang, C. L., Wang, J., Zhang, X., Zhao, F. S., Hu, W. H., et al. (2011). Using ABCG2-molecule-expressing side population cells to identify cancer stem-like cells in a human ovarian cell line. Cell Biology International, 35(3), 227–234.
He, J., Liu, Y., Zhu, T., Zhu, J., Dimeco, F., Vescovi, A. L., et al. (2012). CD90 is identified as a marker for cancer stem cells in primary high-grade gliomas using tissue microarrays. Molecular & Cellular Proteomics, 11(6), M111.010744.
Kumar, S. M., Liu, S., Lu, H., Zhang, H., Zhang, P. J., Gimotty, P. A., et al. (2012). Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene, 31(47), 4898–4911.
Balber, A. E. (2011). Concise review: Aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues; characteristics, activities, and emerging uses in regenerative medicine. Stem Cells, 29, 570–575.
Chen, S. Y., Huang, Y. C., Liu, S. P., Tsai, F. J., Shyu, W. C., & Lin, S. Z. (2011). An overview of concepts for cancer stem cells. Cell Transplantation, 20, 113–120.
Han, M. E., & Oh, S. O. (2013). Gastric stem cells and gastric cancer stem cells. Anatomy & Cell Biology, 46(1), 8–18.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhavan-Niaki, H., Samadani, A.A. Molecular Insight in Gastric Cancer Induction: An Overview of Cancer Stemness Genes. Cell Biochem Biophys 68, 463–473 (2014). https://doi.org/10.1007/s12013-013-9749-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9749-7