Abstract
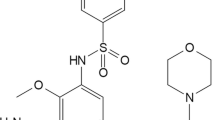
To examine the antiproliferative effect of the combination of docetaxel and sorafenib, applied to the representative non-small cell lung cancer cell line A549 cells either wild type or with acquired resistance to docetaxel (A549/D). The aim of this study is to evaluate the synergistic effect of combination treatment on cell growth inhibition and to elucidate the involved molecular mechanisms. A549 cells with acquired resistance to docetaxel were established by continuous exposure to docetaxel. We examined the effect of different combinatorial treatment on cell proliferation and cell cycle distribution. In addition, the effect of combinatorial treatments on proliferative and apoptotic signalling pathway were studied. Our results showed that the synergistic effect presented when A549 cells were treated with docetaxel followed by sorafenib or when A549/D cells were treated in reverse sequence. Furthermore, we suggested that synergistic effect in A549/D cells was caused by inhibiting P-gp function and altering in the balance of growth and apoptotic signalling pathways. Our data suggested a potential role of sorafenib in chemosensitizing docetaxel-resistant cancer cells. This study also provides molecular evidence for applying different therapeutic strategies for patients with different genetic and proteomic profile.




Similar content being viewed by others
References
Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., & Thun, M. J. (2009). Cancer statistics, 2009. CA Cancer Journal for Clinicians, 59(4), 225–249.
Society AC. (2009). Cancer facts and figures. Atlanta: American Cancer Society, Inc.
Schiller, J. H., Harrington, D., Belani, C. P., Langer, C., Sandler, A., Krook, J., et al. (2002). Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. The New England Journal of Medicine, 346(2), 92–98.
Wilhelm, S., & Chien, D. S. (2002). BAY 43-9006: preclinical data. Current Pharmaceutical Design, 8(25), 2255–2257.
Wilhelm, S. M., Carter, C., Tang, L., Wilkie, D., McNabola, A., Rong, H., et al. (2004). BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Research, 64(19), 7099–7109.
Rahmani, M., Davis, E. M., Bauer, C., Dent, P., & Grant, S. (2005). Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. The Journal of Biological Chemistry, 280(42), 35217–35227.
Takezawa, K., Okamoto, I., Yonesaka, K., Hatashita, E., Yamada, Y., Fukuoka, M., et al. (2009). Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Research, 69(16), 6515–6521.
Liu, L., Cao, Y., Chen, C., Zhang, X., McNabola, A., Wilkie, D., et al. (2006). Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Research, 66(24), 11851–11858.
Yu, C., Bruzek, L. M., Meng, X. W., Gores, G. J., Carter, C. A., Kaufmann, S. H., et al. (2005). The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene, 24(46), 6861–6869.
Carter, C. A., Chen, C., Brink, C., Vincent, P., Maxuitenko, Y. Y., Gilbert, K. S., et al. (2007). Sorafenib is efficacious and tolerated in combination with cytotoxic or cytostatic agents in preclinical models of human non-small cell lung carcinoma. Cancer Chemotherapy and Pharmacology, 59(2), 183–195.
Ulivi, P., Arienti, C., Zoli, W., Scarsella, M., Carloni, S., Fabbri, F., et al. (2010). In vitro and in vivo antitumor efficacy of docetaxel and sorafenib combination in human pancreatic cancer cells. Current Cancer Drug Targets, 10(6), 600–610.
Bareford, M. D., Hamed, H. A., Tang, Y., Cruickshanks, N., Burow, M. E., Fisher, P. B., et al. (2011). Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells. Autophagy, 7(10), 1261–1262.
Pasqualetti, G., Ricciardi, S., Mey, V., Del Tacca, M., & Danesi, R. (2011). Synergistic cytotoxicity, inhibition of signal transduction pathways and pharmacogenetics of sorafenib and gemcitabine in human NSCLC cell lines. Lung Cancer, 74(2), 197–205.
Flaherty, K. T., Schiller, J., Schuchter, L. M., Liu, G., Tuveson, D. A., Redlinger, M., et al. (2008). A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clinical Cancer Research, 14(15), 4836–4842.
Scagliotti, G., Novello, S., von Pawel, J., Reck, M., Pereira, J. R., Thomas, M., et al. (2010). Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. Journal of Clinical Oncology, 28(11), 1835–1842.
Okamoto, I., Miyazaki, M., Morinaga, R., Kaneda, H., Ueda, S., Hasegawa, Y., et al. (2010). Phase I clinical and pharmacokinetic study of sorafenib in combination with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Investigational New Drugs, 28(6), 844–853.
Montero, A., Fossella, F., Hortobagyi, G., & Valero, V. (2005). Docetaxel for treatment of solid tumours: a systematic review of clinical data. The Lancet Oncology, 6(4), 229–239.
Pan, F., Tian, J., Zhang, X., Zhang, Y., & Pan, Y. (2011). Synergistic interaction between sunitinib and docetaxel is sequence dependent in human non-small lung cancer with EGFR TKIs-resistant mutation. Journal of Cancer Research and Clinical Oncology, 137(9), 1397–1408.
Herbst, R. S., Sun, Y., Eberhardt, W. E., Germonpre, P., Saijo, N., Zhou, C., et al. (2010). Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. The Lancet Oncology, 11(7), 619–626.
Heymach, J. V., Johnson, B. E., Prager, D., Csada, E., Roubec, J., Pesek, M., et al. (2007). Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. Journal of clinical oncology, 25(27), 4270–4277.
Lo Nigro, C., Maffi, M., Fischel, J. L., Formento, P., Milano, G., & Merlano, M. (2008). The combination of docetaxel and the somatostatin analogue lanreotide on androgen-independent docetaxel-resistant prostate cancer: experimental data. BJU International, 102(5), 622–627.
Chou, T. C., & Talalay, P. (1984). Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation, 22, 27–55.
Cheng, H., An, S. J., Zhang, X. C., Dong, S., Zhang, Y. F., Chen, Z. H., et al. (2011). In vitro sequence-dependent synergism between paclitaxel and gefitinib in human lung cancer cell lines. Cancer Chemotherapy and Pharmacology, 67(3), 637–646.
Jordan, M. A., & Wilson, L. (2004). Microtubules as a target for anticancer drugs. Nature Reviews Cancer, 4(4), 253–265.
Morelli, M. P., Cascone, T., Troiani, T., De Vita, F., Orditura, M., Laus, G., et al. (2005). Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Annals of Oncology, 16(4), iv61–iv68.
Conrad, C., Ischenko, I., Kohl, G., Wiegand, U., Guba, M., Yezhelyev, M., et al. (2007). Antiangiogenic and antitumor activity of a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor ZD6474 in a metastatic human pancreatic tumor model. Anti-Cancer Drugs, 18(5), 569–579.
Mahaffey, C. M., Davies, A. M., Lara, P. N, Jr, Pryde, B., Holland, W., Mack, P. C., et al. (2007). Schedule-dependent apoptosis in K-ras mutant non-small-cell lung cancer cell lines treated with docetaxel and erlotinib: rationale for pharmacodynamic separation. Clinical Lung Cancer, 8(9), 548–553.
Sarkar, S., Mazumdar, A., Dash, R., Sarkar, D., Fisher, P. B., & Mandal, M. (2011). ZD6474 enhances paclitaxel antiproliferative and apoptotic effects in breast carcinoma cells. Journal of Cellular Physiology, 226(2), 375–384.
McHugh, L. A., Kriajevska, M., Mellon, J. K., & Griffiths, T. R. (2007). Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens–evidence of schedule-dependent synergy. Urology, 69(2), 390–394.
Monteverde, M., Tonissi, F., Fischel, J. L., Etienne-Grimaldi, M. C., Milano, G., Merlano, M., et al. (2011). Combination of docetaxel and vandetanib in docetaxel-sensitive or resistant PC3 cell line. Urologic Oncology, 31(6), 776–786.
Collins, D. M., Crown, J., O’Donovan, N., Devery, A., O’Sullivan, F., O’Driscoll, L., et al. (2010). Tyrosine kinase inhibitors potentiate the cytotoxicity of MDR-substrate anticancer agents independent of growth factor receptor status in lung cancer cell lines. Investigational New Drugs, 28(4), 433–444.
Flaig, T. W., Su, L. J., McCoach, C., Li, Y., Raben, D., Varella-Garcia, M., et al. (2009). Dual epidermal growth factor receptor and vascular endothelial growth factor receptor inhibition with vandetanib sensitizes bladder cancer cells to cisplatin in a dose- and sequence-dependent manner. BJU International, 103(12), 1729–1737.
Perez-Soler, R., Piperdi, B., Haigentz, M., Ling, Y-H. (2004). Determinants of sensitivity to the EGFR TK inhibitor erlotinib (E) in a panel of NSCLC cell lines. Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition), 22 (14), 7026.
Li, T., Ling, Y. H., Goldman, I. D., & Perez-Soler, R. (2007). Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clinical Cancer Research, 13(11), 3413–3422.
Wang, T., Wei, J., Qian, X., Ding, Y., Yu, L., & Liu, B. (2008). Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Letters, 262(2), 214–222.
Yamanaka, K., Nakata, M., Kaneko, N., Fushiki, H., Kita, A., Nakahara, T., et al. (2011). YM155, a selective survivin suppressant, inhibits tumor spread and prolongs survival in a spontaneous metastatic model of human triple negative breast cancer. International Journal of Oncology, 39(3), 569–575.
Nakahara, T., Yamanaka, K., Hatakeyama, S., Kita, A., Takeuchi, M., Kinoyama, I., et al. (2011). YM155, a novel survivin suppressant, enhances taxane-induced apoptosis and tumor regression in a human Calu 6 lung cancer xenograft model. Anti-Cancer Drugs, 22(5), 454–462.
Yoon, D. H., Shin, J. S., Jin, D. H., Hong, S. W., Jung, K. A., Kim, S. M., et al. (2012). The survivin suppressant YM155 potentiates chemosensitivity to gemcitabine in the human pancreatic cancer cell line MiaPaCa-2. Anticancer Research, 32(5), 1681–1688.
Ling, X., Bernacki, R. J., Brattain, M. G., & Li, F. (2004). Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. The Journal of Biological Chemistry, 279(15), 15196–15203.
Uchida, H., Tanaka, T., Sasaki, K., Kato, K., Dehari, H., Ito, Y., et al. (2004). Adenovirus-mediated transfer of siRNA against survivin induced apoptosis and attenuated tumor cell growth in vitro and in vivo. Molecular Therapy, 10(1), 162–171.
Kirkin, V., Joos, S., & Zornig, M. (2004). The role of Bcl-2 family members in tumorigenesis. Biochimica Biophysica Acta, 1644(2–3), 229–249.
Ciardiello, F., Caputo, R., Borriello, G., Del Bufalo, D., Biroccio, A., Zupi, G., et al. (2002). ZD1839 (IRESSA), an EGFR-selective tyrosine kinase inhibitor, enhances taxane activity in bcl-2 overexpressing, multidrug-resistant MCF-7 ADR human breast cancer cells. International Journal of Cancer, 98(3), 463–469.
Maraz, A., Furak, J., Palfoldi, R., Eller, J., Szanto, E., Kahan, Z., et al. (2011). Roles of BCL-2 and MDR1 expression in the efficacy of paclitaxel-based lung cancer chemoradiation. Anticancer Research, 31(4), 1431–1436.
Tan, N., Malek, M., Zha, J., Yue, P., Kassees, R., Berry, L., et al. (2011). Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clinical Cancer Research, 17(6), 1394–1404.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, X., Zhang, T. Alteration in the Balance of Prosurvival and Proapoptotic Signalling Pathways Leads to Sequence-Dependent Synergism Between Docetaxel and Sorafenib in Human Non-small Cell Lung Cancer Cell Lines. Cell Biochem Biophys 68, 411–418 (2014). https://doi.org/10.1007/s12013-013-9722-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9722-5