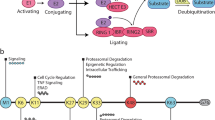
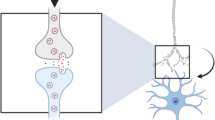
Abstract
Neurons have highly specialized intracellular compartments that facilitate the development and activity of the nervous system. Ubiquitination is a post-translational modification that controls many aspects of neuronal function by regulating protein abundance. Disruption of this signaling pathway has been demonstrated in neurological disorders such as Parkinson’s disease, Amyotrophic Lateral Sclerosis and Angleman Syndrome. Since many neurological disorders exhibit ubiquitinated protein aggregates, the loss of neuronal ubiquitin homeostasis may be an important contributor of disease. This review discusses the mechanisms utilized by neurons to control the free pool of ubiquitin necessary for normal nervous system development and function as well as new roles of protein ubiquitination in regulating the synaptic activity.


Similar content being viewed by others
References
Yi, J. J., & Ehlers, M. D. (2007). Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacological Reviews, 59, 14–39.
DiAntonio, A., Haghighi, A. P., Portman, S. L., Lee, J. D., Amaranto, A. M., et al. (2001). Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature, 412, 449–452.
Ding, M., Chao, D., Wang, G., & Shen, K. (2007). Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science, 317, 947–951.
Burbea, M., Dreier, L., Dittman, J. S., Grunwald, M. E., & Kaplan, J. M. (2002). Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C-elegans. Neuron, 35, 107–120.
Colledge, M., Snyder, E. M., Crozier, R. A., Soderling, J. A., Jin, Y., et al. (2003). Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron, 40, 595–607.
Bizzi, A., Schaetzle, B., Patton, A., Gambetti, P., & Autilio-Gambetti, L. (1991). Axonal transport of two major components of the ubiquitin system: free ubiquitin and ubiquitin carboxyl-terminal hydrolase PGP 9.5. Brain Research, 548, 292–299.
Goldstein, G., Scheid, M., Hammerling, U., Schlesinger, D. H., Niall, H. D., et al. (1975). Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proceedings of the National Academy of Sciences of the United States of America, 72, 11–15.
Wilkinson, K. D., Urban, M. K., & Haas, A. L. (1980). Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. Journal of Biological Chemistry, 255, 7529–7532.
Hershko, A., Eytan, E., Ciechanover, A., & Haas, A. L. (1982). Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. Journal of Biological Chemistry, 257, 13964–13970.
Ciehanover, A., Hod, Y., & Hershko, A. (1978). A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochemical and Biophysical Research Communications, 81, 1100–1105.
Kaiser, S. E., Riley, B. E., Shaler, T. A., Trevino, R. S., Becker, C. H., et al. (2011). Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nature Methods, 8, 691–696.
Fornace, A. J, Jr, Alamo, I, Jr, Hollander, M. C., & Lamoreaux, E. (1989). Ubiquitin mRNA is a major stress-induced transcript in mammalian cells. Nucleic Acids Research, 17, 1215–1230.
Bond, U., & Schlesinger, M. J. (1986). The chicken ubiquitin gene contains a heat shock promoter and expresses an unstable mRNA in heat-shocked cells. Molecular and Cellular Biology, 6, 4602–4610.
Wilkinson, K. D., Tashayev, V. L., O’Connor, L. B., Larsen, C. N., Kasperek, E., et al. (1995). Metabolism of the polyubiquitin degradation signal: Structure, mechanism, and role of isopeptidase T. Biochemistry, 34, 14535–14546.
Ryu, K. Y., Garza, J. C., Lu, X. Y., Barsh, G. S., & Kopito, R. R. (2008). Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proceedings of the National Academy of Sciences of the United States of America, 105, 4016–4021.
Ryu, K. Y., Maehr, R., Gilchrist, C. A., Long, M. A., Bouley, D. M., et al. (2007). The mouse polyubiquitin gene UbC is essential for fetal liver development, cell-cycle progression and stress tolerance. EMBO Journal, 26, 2693–2706.
Ryu, K. Y., Sinnar, S. A., Reinholdt, L. G., Vaccari, S., Hall, S., et al. (2008). The mouse polyubiquitin gene Ubb is essential for meiotic progression. Molecular and Cellular Biology, 28, 1136–1146.
Ryu, K. Y., Park, H., Rossi, D. J., Weissman, I. L., & Kopito, R. R. (2012). Perturbation of the hematopoietic system during embryonic liver development due to disruption of polyubiquitin gene Ubc in mice. PLoS ONE, 7, e32956.
Haas, A. L., Warms, J. V., Hershko, A., & Rose, I. A. (1982). Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. Journal of Biological Chemistry, 257, 2543–2548.
Hershko, A., Heller, H., Elias, S., & Ciechanover, A. (1983). Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. Journal of Biological Chemistry, 258, 8206–8214.
Pickart, C. M., & Rose, I. A. (1985). Functional heterogeneity of ubiquitin carrier proteins. Journal of Biological Chemistry, 260, 1573–1581.
Eletr, Z. M., Huang, D. T., Duda, D. M., Schulman, B. A., & Kuhlman, B. (2005). E2 conjugating enzymes must disengage from their E1 enzymes before E3-dependent ubiquitin and ubiquitin-like transfer. Nature Structural & Molecular Biology, 12, 933–934.
Clague, M. J., Liu, H., & Urbe, S. (2012). Governance of endocytic trafficking and signaling by reversible ubiquitylation. Developmental Cell, 23, 457–467.
Chau, V., Tobias, J. W., Bachmair, A., Marriott, D., Ecker, D. J., et al. (1989). A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science, 243, 1576–1583.
Finley, D., Sadis, S., Monia, B. P., Boucher, P., Ecker, D. J., et al. (1994). Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Molecular and Cellular Biology, 14, 5501–5509.
Thrower, J. S., Hoffman, L., Rechsteiner, M., & Pickart, C. M. (2000). Recognition of the polyubiquitin proteolytic signal. EMBO Journal, 19, 94–102.
Abbott, D. W., Yang, Y., Hutti, J. E., Madhavarapu, S., Kelliher, M. A., et al. (2007). Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Molecular and Cellular Biology, 27, 6012–6025.
Chan, N. L., & Hill, C. P. (2001). Defining polyubiquitin chain topology. Natural Structural Biology, 8, 650–652.
Reggiori, F., & Pelham, H. R. B. (2001). Sorting of proteins into multivesicular bodies: Ubiquitin-dependent and -independent targeting. EMBO Journal, 20, 5176–5186.
Sigismund, S., Woelk, T., Puri, C., Maspero, E., Tacchetti, C., et al. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proceedings of the National Academy of Sciences of the United States of America, 102, 2760–2765.
Kravtsova-Ivantsiv, Y., & Ciechanover, A. (2012). Non-canonical ubiquitin-based signals for proteasomal degradation. Journal of Cell Science, 125, 539–548.
Bence, N. F., Sampat, R. M., & Kopito, R. R. (2001). Impairment of the ubiquitin-proteasome system by protein aggregation. Science, 292, 1552–1555.
Upadhya, S. C., & Hegde, A. N. (2007). Role of the ubiquitin proteasome system in Alzheimer’s disease. BMC Biochemistry, 8(Suppl 1), S12.
Dawson, T. M., & Dawson, V. L. (2003). Molecular pathways of neurodegeneration in Parkinson’s disease. Science, 302, 819–822.
Osaka, H., Wang, Y. L., Takada, K., Takizawa, S., Setsuie, R., et al. (2003). Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Human Molecular Genetics, 12, 1945–1958.
Anderson, C., Crimmins, S., Wilson, J. A., Korbel, G. A., Ploegh, H. L., et al. (2005). Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. Journal of Neurochemistry, 95, 724–731.
Shabek, N., & Ciechanover, A. (2010). Degradation of ubiquitin: The fate of the cellular reaper. Cell Cycle, 9, 523–530.
Leroy, E., Boyer, R., Auburger, G., Leube, B., Ulm, G., et al. (1998). The ubiquitin pathway in Parkinson’s disease. Nature, 395, 451–452.
Saigoh, K., Wang, Y. L., Suh, J. G., Yamanishi, T., Sakai, Y., et al. (1999). Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nature Genetics, 23, 47–51.
Ehlers, M. D. (2003). Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nature Neuroscience, 6, 231–242.
Sakurai, M., Sekiguchi, M., Zushida, K., Yamada, K., Nagamine, S., et al. (2008). Reduction in memory in passive avoidance learning, exploratory behaviour and synaptic plasticity in mice with a spontaneous deletion in the ubiquitin C-terminal hydrolase L1 gene. European Journal of Neuroscience, 27, 691–701.
Cartier, A. E., Djakovic, S. N., Salehi, A., Wilson, S. M., Masliah, E., et al. (2009). Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. Journal of Neuroscience, 29, 7857–7868.
Borodovsky, A., Kessler, B. M., Casagrande, R., Overkleeft, H. S., Wilkinson, K. D., et al. (2001). A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO Journal, 20, 5187–5196.
Hu, M., Li, P., Song, L., Jeffrey, P. D., Chenova, T. A., et al. (2005). Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO Journal, 24, 3747–3756.
Lee, B. H., Lee, M. J., Park, S., Oh, D. C., Elsasser, S., et al. (2010). Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature, 467, 179–184.
Wilson, S. M., Bhattacharyya, B., Rachel, R. A., Coppola, V., Tessarollo, L., et al. (2002). Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nature Genetics, 32, 420–425.
Chen, P. C., Qin, L. N., Li, X. M., Walters, B. J., Wilson, J. A., et al. (2009). The proteasome-associated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. Journal of Neuroscience, 29, 10909–10919.
Crimmins, S., Jin, Y., Wheeler, C., Huffman, A. K., Chapman, C., et al. (2006). Transgenic rescue of ataxia mice with neuronal-specific expression of ubiquitin-specific protease 14. Journal of Neuroscience, 26, 11423–11431.
Chen, P. C., Bhattacharyya, B. J., Hanna, J., Minkel, H., Wilson, J. A., et al. (2011). Ubiquitin homeostasis is critical for synaptic development and function. Journal of Neuroscience, 31, 17505–17513.
Hanna, J., Meides, A., Zhang, D. P., & Finley, D. (2007). A ubiquitin stress response induces altered proteasome composition. Cell, 129, 747–759.
Bingol, B., & Sheng, M. (2011). Deconstruction for reconstruction: The role of proteolysis in neural plasticity and disease. Neuron, 69, 22–32.
Chen, H., Polo, S., Di Fiore, P. P., & De Camilli, P. V. (2003). Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proceedings of the National Academy of Sciences of the United States of America, 100, 14908–14913.
Rinetti, G. V., & Schweizer, F. E. (2010). Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. Journal of Neuroscience, 30, 3157–3166.
Acknowledgments
This research was supported by the Evelyn F. McKnight Brain Institute, NIH/NINDS Grants NS047533 and NS074456 (S.M.W).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hallengren, J., Chen, PC. & Wilson, S.M. Neuronal Ubiquitin Homeostasis. Cell Biochem Biophys 67, 67–73 (2013). https://doi.org/10.1007/s12013-013-9634-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9634-4