Abstract
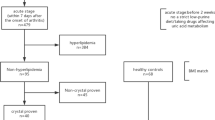
Gout patients have a high incidence of atherosclerotic coronary heart disease. Low serum paraoxonase (PON) activity is considered a risk factor for atherosclerosis. The relationships among paraoxonase-1 (PON1) activity, oxidative stress parameters, and atherosclerosis in gout is not known. Therefore, we determined the plasma levels of malondialdehyde (MDA), oxidized low-density lipoprotein (Ox-LDL), and activities of PON1/superoxide dismutase (SOD) activities in 49 gout patients (mean age 44.2 ± 7.0 years) and 42 healthy, age-matched controls (mean age 45.0 ± 9.3 years). PON1 was measured spectrophotometrically, MDA by thiobarbituric acid method, SOD by Griess reaction, and Ox-LDL by sandwich ELISA. Lipid and other biochemical parameters were determined by routine laboratory methods. In gout patients, PON1/SOD activities and MDA/Ox-LDL levels were 131.3 ± 25.3/75.3 ± 28.9 kU l−1 and 6.12 ± 1.67 nmol ml−1/690.1 ± 180.2 μg l−1, respectively. In controls, these were 172.5 ± 27.8/94.0 ± 26.3 kU l−1 and 4.10 ± 1.25 nmol ml−1/452.3 ± 152.1 μg l−1, respectively. Thus, in gout patients, there was a significant decrease in PON1 (P < 0.01) and SOD (P < 0.05) activities, and an increase in MDA (P < 0.01) and Ox-LDL (P < 0.01) levels compared with controls. PON1 activity correlated positively with SOD (P < 0.05), and negatively with MDA (P < 0.01) and Ox-LDL (P < 0.01). These results suggest that gout patients were in a state of oxidative stress and the protective effects of HDL against atherosclerosis maybe dependent on PON1 activity. These findings may explain in part the reported increase in cardiovascular mortality in gout patients.
Similar content being viewed by others
References
Choi, H. K., Mount, D. B., & Reginato, A. M. (2005). Pathogenesis of gout. Annals of Internal Medicine, 143, 499–516.
Krishnan, E., Svendsen, K., Neaton, J. D., Grandits, G., & Kuller, L. H. (2008). Long-term cardiovascular mortality among middle-aged men with gout. Archives of Internal Medicine, 168, 1104–1110.
Takahashi, S., Yamamoto, T., Moriwaki, Y., Tsutsumi, Z., & Higashino, K. (1995). Increased concentrations of serum Lp(a) lipoprotein in patients with primary gout. Annals of the Rheumatic Diseases, 54, 90–93.
Mak, A., Ho, R. C. M., Tan, J. Y. S., et al. (2009). Atherogenic serum lipid profile is an independent predictor for gouty flares in patients with gouty arthropathy. Rheumatology, 48, 262–265.
Cheng, T. T., Lai, H. M., Chang, H. W., & Luo, S. F. (2005). Elevated serum homocysteine levels for gouty patients. Clinical Rheumatology, 24, 103–106.
Krishnan, E. (2010). Inflammation, oxidative stress and lipids: The risk triad for atherosclerosis in gout. Rheumatology, 3, 1–10.
Madamanchi, N. R., Vendrov, A., & Runge, M. S. (2005). Oxidative stress and vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 29–38.
Hahn, M., & Subbiah, M. T. (1994). Significant association of lipid peroxidation products with high density lipoproteins. Biochemistry and Molecular Biology International, 33, 699–704.
Steinberg, D. (1997). Low density lipoprotein oxidation and its pathobiological significance. The Journal of Biological Chemistry, 272, 20963–20966.
Navab, M., Anantharamaiah, G. M., Reddy, S. T., Van Lenten, B. J., Ansell, B. J., & Fogelman, A. M. (2006). Mechanisms of disease: Proatherogenic HDL—an evolving field. Nature Clinical Practice Endocrinology & Metabolism, 2, 504–511.
Mackness, M., Durrington, P., & Mackness, B. (2004). Paraoxonase-1 activity, concentration and genotype in cardiovascular disease. Current Opinion in Lipidology, 15, 399–404.
Mackness, B., Davies, G. K., Turkie, W., et al. (2001). Paraoxonase status in coronary heart disease: Are activity and concentration more important than genotype? Arteriosclerosis, Thrombosis, and Vascular Biology, 21, 1451–1457.
Esterbauer, H., Schaur, R. J., & Zollner, H. (1991). Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radical Biology and Medicine, 11, 81–128.
Beckman, J. S., & Koppenol, W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. American Journal of Physiology, 271, C3424–3437.
Wallace, S. L., Robinson, H., Masi, A. T., Decker, J. L., McCarty, D. J., & Yu, T. F. (1997). Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and Rheumatism, 20, 895–900.
Ferre, N., Camps, J., Prats, E., et al. (2008). Serum paraoxonase activity: A new additional test for the improved evaluation of chronic liver damage. Clinical Chemistry, 48, 261–268.
Aviram, M., Rosenblat, M., Billecke, S., et al. (1999). Human serum paraoxonase (PON1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radical Biology and Medicine, 26, 892–904.
Nakamura, K., Endo, H., & Kashiwazaki, S. (1987). Serum oxidation activities and rheumatoid arthritis. International Journal of Tissue Reactions, 9, 307–316.
Mackness, B., Hunt, R., Durrington, P. N., & Mackness, M. I. (1997). Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 17, 1233–1238.
Feingold, K. R., Memon, R. A., Moser, A. H., & Grunfeld, C. (1998). Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis, 139, 307–315.
Mackness, M. I., Arrol, S., & Durrington, P. N. (1991). Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Letters, 286, 152–154.
Inokuchi, T., Moriwaki, Y., Tsutsui, H., et al. (2006). Plasma interleukin (IL)-18 (interferon-gamma-inducing factor) and other inflammatory cytokines in patients with gouty arthritis and monosodium urate monohydrate crystal-induced secretion of IL-18. Cytokine, 33, 21–27.
Kumon, Y., Nakauchi, Y., Suehiro, T., et al. (2002). Proinflammatory cytokines but not acute phase serum amyloid A or C-reactive protein, downregulate paraoxonase 1 (PON1) expression by HepG2 cells. Amyloid, 9, 160–164.
James, R. W., & Deakin, S. P. (2004). The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radical Biology and Medicine, 37, 1986–1994.
Deakin, S., Leviev, I., Gomaraschi, M., Calabresi, L., Franceschini, G., & James, R. W. (2002). Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. The Journal of Biological Chemistry, 277, 4301–4308.
Rashid, S., Uffelman, K. D., & Lewis, G. F. (2002). The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. Journal of Diabetes Complications, 16, 24–28.
Roubenoff, R., Klag, M. J., Mead, L. A., Liang, K. Y., Seidler, A. J., & Hochberg, M. C. (1991). Incidence and risk factors for gout in white men. The Journal of the American Medical Association, 266, 3004–3007.
Patterson, R. A., Horsley, E. T., & Leake, D. S. (2003). Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: Important role of uric acid. Journal of Lipid Research, 44, 512–521.
Abuja, P. M. (1999). Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Letters, 446, 305–308.
Muraoka, S., & Miura, T. (2003). Inhibition by uric acid of free radicals that damage biological molecules. Pharmacology and Toxicology, 93, 284–289.
Maples, K. R., & Mason, R. P. (1988). Free radical metabolite of uric acid. The Journal of Biological Chemistry, 263, 1709–1712.
Santos, C. X., Anjos, E. I., & Augusto, O. (1999). Uric acid oxidation by peroxynitrite: Multiple reactions, free radical formation, and amplification of lipid oxidation. Archives of Biochemistry and Biophysics, 372, 285–294.
Sautin, Y. Y., Nakagawa, T., Zharikov, S., & Johnson, R. J. (2007). Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase mediated oxidative/nitrosative stress. American Journal of Physiology, 293, C584–C596.
Bartesaghi, S., Ferrer-Sueta, G., Peluffo, G., et al. (2007). Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids, 32, 501–515.
Bagnati, M., Perugini, C., Cau, C., Bordone, R., Albano, E., & Bellomo, G. (1999). When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: A study using uric acid. Biochemical Journal, 340, 143–152.
Acknowledgment
The authors thank the Department of Health of Sichuan Province for the financial support (Grant #20090143), for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xing-Liang Jiang and Min Li contributed equally to this work as co-first authors.
Rights and permissions
About this article
Cite this article
Jiang, XL., Li, M., Zhou, JG. et al. Plasma Paraoxonase-1, Oxidized Low-Density Lipoprotein and Lipid Peroxidation Levels in Gout Patients. Cell Biochem Biophys 61, 461–466 (2011). https://doi.org/10.1007/s12013-011-9221-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9221-5