Abstract
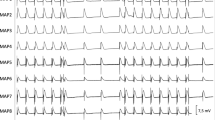
Ivabradine has recently been demonstrated to have antiarrhythmic properties in atrial fibrillation. The aim of the present study was to assess the electrophysiologic profile of ivabradine in an experimental whole-heart model of long-QT-syndrome. In 12 isolated rabbit hearts long-QT-2-syndrome (LQT2) was simulated by infusion of d,l-sotalol (100 µM). 12 rabbit hearts were treated with veratridine (0.5 µM) to mimic long-QT-3-syndrome (LQT3). Sotalol induced a significant prolongation of QT-interval (+ 40 ms, p < 0.01) and action potential duration (APD, + 20 ms, p < 0.01). Similar results were obtained in veratridine-treated hearts (QT-interval: +52 ms, p < 0.01; APD: + 41 ms, p < 0.01). Of note, both sotalol (+ 26 ms, p < 0.01) and veratridine (+ 42 ms, p < 0.01) significantly increased spatial dispersion of repolarisation. Additional infusion of ivabradine (5 µM) did not change these parameters in sotalol-pretreated hearts but resulted in a further significant increase of QT-interval (+ 26 ms, p < 0.05) and APD (+ 49 ms, p < 0.05) in veratridine-treated hearts. Lowering of potassium concentration in bradycardic AV-blocked hearts resulted in the occurrence of early afterdepolarizations (EAD) or polymorphic ventricular tachycardias (VT) resembling torsade de pointes in 6 of 12 sotalol-treated hearts (56 episodes) and 6 of 12 veratridine-treated hearts (73 episodes). Additional infusion of ivabradine increased occurrence of polymorphic VT. Ivabradine treatment resulted in occurrence of EAD and polymorphic VT in 9 of 12 sotalol-treated hearts (212 episodes), and 8 of 12 veratridine-treated hearts (155 episodes). Treatment with ivabradine in experimental models of LQT2 and LQT3 increases proarrhythmia. A distinct interaction with potassium currents most likely represents a major underlying mechanism. These results imply that ivabradine should be employed with caution in the presence of QT-prolongation.




Similar content being viewed by others
References
El Chemaly, A., Magaud, C., Patri, S., Jayle, C., Guinamard, R., & Bois, P. (2007). The heart rate-lowering agent ivabradine inhibits the pacemaker current I(f) in human atrial myocytes. Journal of Cardiovascular Electrophysiology, 18, 1190–1196.
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G., Coats, A. J., et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal, 37, 2129–2200.
Melgari, D., Brack, K. E., Zhang, C., Zhang, Y., El Harchi, A., Mitcheson, J. S., et al. (2015). hERG potassium channel blockade by the HCN channel inhibitor bradycardic agent ivabradine. Journal of the American Heart Association, 4(4), e001813.
Lees-Miller, J. P., Guo, J., Wang, Y., Perissinotti, L. L., Noskov, S. Y., & Duff, H. J. (2015). Ivabradine prolongs phase 3 of cardiac repolarization and blocks the hERG1 (KCNH2) current over a concentration-range overlapping with that required to block HCN4. Journal of Molecular and Cellular Cardiology, 85, 71–78.
Frommeyer, G., Weller, J., Ellermann, C., Bogeholz, N., Leitz, P., Dechering, D. G., et al. (2017). Ivabradine reduces digitalis-induced ventricular arrhythmias. Basic & Clinical Pharmacology & Toxicology, 121, 526–530.
Frommeyer, G., Weller, J., Ellermann, C., Kaese, S., Kochhauser, S., Lange, P. S., et al. (2017). Antiarrhythmic properties of ivabradine in an experimental model of Short-QT-Syndrome. Clinical and Experimental Pharmacology & Physiology, 44, 941–945.
Frommeyer, G., Sterneberg, M., Dechering, D. G., Ellermann, C., Bogeholz, N., Kochhauser, S., et al. (2017). Effective suppression of atrial fibrillation by ivabradine: Novel target for an established drug? International Journal of Cardiology, 236, 237–243.
Verrier, R. L., Bonatti, R., Silva, A. F., Batatinha, J. A., Nearing, B. D., Liu, G., et al. (2014). If inhibition in the atrioventricular node by ivabradine causes rate-dependent slowing of conduction and reduces ventricular rate during atrial fibrillation. Heart Rhythm, 11, 2288–2296.
Verrier, R. L., Silva, A. F., Bonatti, R., Batatinha, J. A., Nearing, B. D., Liu, G., et al. (2015). Combined actions of ivabradine and ranolazine reduce ventricular rate during atrial fibrillation. Journal of Cardiovascular Electrophysiology, 26, 329–335.
Mengesha, H. G., Weldearegawi, B., Petrucka, P., Bekele, T., Otieno, M. G., & Hailu, A. (2017). Effect of ivabradine on cardiovascular outcomes in patients with stable angina: Meta-analysis of randomized clinical trials. BMC Cardiovascular Disorders, 17, 105.
Frommeyer, G., Clauss, C., Ellermann, C., Bogossian, H., Dechering, D. G., Kochhauser, S., et al. (2017). Antiarrhythmic effect of vernakalant in an experimental model of Long-QT-syndrome. Europace, 19, 866–873.
Frommeyer, G., Garthmann, J., Ellermann, C., Dechering, D. G., Kochhauser, S., Reinke, F., et al. (2017). Broad antiarrhythmic effect of mexiletine in different arrhythmia models. Europace (in press).
Frommeyer, G., Milberg, P., Witte, P., Stypmann, J., Koopmann, M., Lucke, M., et al. (2011). A new mechanism preventing proarrhythmia in chronic heart failure: Rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure. European Journal of Heart Failure, 13, 1060–1069.
Milberg, P., Frommeyer, G., Kleideiter, A., Fischer, A., Osada, N., Breithardt, G., et al. (2011). Antiarrhythmic effects of free polyunsaturated fatty acids in an experimental model of LQT2 and LQT3 due to suppression of early afterdepolarizations and reduction of spatial and temporal dispersion of repolarization. Heart Rhythm, 8, 1492–1500.
Antzelevitch, C. (2007). Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace, 9(Suppl 4), iv4–i15.
Verduyn, S. C., Vos, M. A., van der Zande, J., Kulcsar, A., & Wellens, H. J. (1997). Further observations to elucidate the role of interventricular dispersion of repolarization and early afterdepolarizations in the genesis of acquired torsade de pointes arrhythmias: A comparison between almokalant and d-sotalol using the dog as its own control. Journal of the American College of Cardiology, 30, 1575–1584.
Thomsen, M. B., Verduyn, S. C., Stengl, M., Beekman, J. D., de Pater, G., van Opstal, J., et al. (2004). Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation, 110, 2453–2459.
van Opstal, J. M., Schoenmakers, M., Verduyn, S. C., de Groot, S. H., Leunissen, J. D., van Der Hulst, F. F., et al. (2001). Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation, 104, 2722–2727.
Roden, D. M. (1998). Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing and Clinical Electrophysiology, 21, 1029–1034.
Frommeyer, G., Fischer, C., Ellermann, C., Dechering, D. G., Kochhauser, S., Lange, P. S., et al. (2018). Additive proarrhythmic effect of combined treatment with QT-prolonging agents. Cardiovascular Toxicology, 18, 84–90.
Acknowledgements
This study was supported by the German Cardiac Society and the Hans und Gertie-Fischer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest related to this study.
Additional information
Handling Editor: Bérengère Dumotier.
Rights and permissions
About this article
Cite this article
Frommeyer, G., Weller, J., Ellermann, C. et al. Ivabradine Aggravates the Proarrhythmic Risk in Experimental Models of Long QT Syndrome. Cardiovasc Toxicol 19, 129–135 (2019). https://doi.org/10.1007/s12012-018-9482-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-9482-y