Abstract
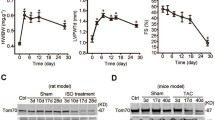
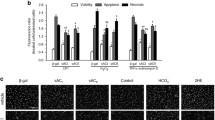
Cytochrome c oxidase (CCO) is a copper-dependent enzyme of mitochondrial respiratory chain. In pressure overload-induced cardiac hypertrophy, copper level and CCO activity are both depressed, along with disturbance in mitochondrial fusion and fission dynamics. Copper repletion leads to recovery of CCO activity and normalized mitochondrial dynamics. The present study was undertaken to define the link between CCO activity and mitochondrial dynamic changes. Primary cultures of neonatal rat cardiomyocytes were treated with phenylephrine to induce cell hypertrophy. Hypertrophic cardiomyocytes were then treated with copper to reverse hypertrophy. In the hypertrophic cardiomyocytes, CCO activity was depressed and mitochondrial fusion was suppressed. Upon copper repletion, CCO activity was recovered and mitochondrial fusion was reestablished. Depression of CCO activity by siRNA targeting CCO assembly homolog 17 (COX17), a copper chaperone for CCO, led to fragmentation of mitochondria, which was not recoverable by copper supplementation. This study thus demonstrates that copper-dependent CCO is critical for mitochondrial fusion in the regression of cardiomyocyte hypertrophy.





Similar content being viewed by others
References
Iwata, S. (1998). Structure and function of bacterial cytochrome c oxidase. Journal of Biochemistry, 123, 369–375.
Abramson, J., Svensson-Ek, M., Byrne, B., & Iwata, S. (2001). Structure of cytochrome c oxidase: A comparison of the bacterial and mitochondrial enzymes. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 1544, 1–9.
Yoshikawa, S., Shinzawa-Itoh, K., & Tsukihara, T. (2000). X-ray structure and the reaction mechanism of bovine heart cytochrome c oxidase. Journal of Inorganic Biochemistry, 82, 1–7.
Saraste, M. (1990). Structural features of cytochrome oxidase. Quarterly Reviews of Biophysics, 23, 331–366.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Itoh, K., et al. (1996). The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science, 272, 1136–1144.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Itoh, K., et al. (1995). Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science, 269, 1069–1074.
Glerum, D. M., Shtanko, A., & Tzagoloff, A. (1996). Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. Journal of Biological Chemistry, 271, 14504–14509.
Takahashi, Y., Kako, K., Kashiwabara, S.-I., Takehara, A., Inada, Y., Arai, H., et al. (2002). Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Molecular and Cellular Biology, 22, 7614–7621.
Leary, S. C., Kaufman, B. A., Pellecchia, G., Guercin, G.-H., Mattman, A., Jaksch, M., et al. (2004). Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Human Molecular Genetics, 13, 1839–1848.
Tzagoloff, A., Capitanio, N., Nobrega, M. P., & Gatti, D. (1990). Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. The EMBO journal, 9, 2759.
Carr, H. S., George, G. N., & Winge, D. R. (2002). Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu (I)-binding protein. Journal of Biological Chemistry, 277, 31237–31242.
Schulze, M., & Rödel, G. (1988). SCO1, a yeast nuclear gene essential for accumulation of mitochondrial cytochrome c oxidase subunit II. Molecular and General Genetics MGG, 211, 492–498.
Nittis, T., George, G. N., & Winge, D. R. (2001). Yeast Sco1, a protein essential for cytochrome c oxidase function Is a Cu (I)-binding protein. Journal of Biological Chemistry, 276, 42520–42526.
Oswald, C., Krause-Buchholz, U., & Rödel, G. (2009). Knockdown of human COX17 Affects assembly and supramolecular organization of cytochrome c oxidase. Journal of Molecular Biology, 389, 470–479.
Wang, B., Dong, D., & Kang, Y. J. (2013). Copper chaperone for superoxide dismutase-1 transfers copper to mitochondria but does not affect cytochrome c oxidase activity. Experimental Biology and Medicine (Maywood, NJ), 238, 1017–1023.
Jiang, Y., Reynolds, C., Xiao, C., Feng, W., Zhou, Z., Rodriguez, W., et al. (2007). Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. Journal of Experimental Medicine, 204, 657–666.
Nishio, M. L., Ornatsky, O. I., Craig, E. E., & Hood, D. A. (1995). Mitochondrial biogenesis during pressure overload induced cardiac hypertrophy in adult rats. Canadian Journal of Physiology and Pharmacology, 73, 630–637.
Müller-Höcker, J., Johannes, A., Droste, M., Kadenbach, B., Pongratz, D., & Hühner, G. (1986). Fatal mitochondrial cardiomyopathy in Kearns-Sayre syndrome with deficiency of cytochrome-c-oxidase in cardiac and skeletal muscle. Virchows Archiv B, 52, 353–367.
Zeviani, M., Van Dyke, D. H., Servidei, S., Bauserman, S. C., Bonilla, E., Beaumont, E. T., et al. (1986). Myopathy and fatal cardiopathy due to cytochrome c oxidase deficiency. Archives of Neurology, 43, 1198–1202.
Buchwald, A., Till, H., Unterberg, C., Oberschmidt, R., Figulla, H. R., & Wiegand, V. (1990). Alterations of the mitochondrial respiratory chain in human dilated cardiomyopathy. European Heart Journal, 11, 509–516.
Elsherif, L., Wang, L., Saari, J. T., & Kang, Y. J. (2004). Regression of dietary copper restriction-induced cardiomyopathy by copper repletion in mice. The Journal of nutrition, 134, 855–860.
Zuo, X., Xie, H., Dong, D., Jiang, N., Zhu, H., & Kang, Y. J. (2010). Cytochrome c oxidase is essential for copper-induced regression of cardiomyocyte hypertrophy. Cardiovascular Toxicology, 10, 208–215.
Dallman, P. R. (1967). Cytochrome oxidase repair during treatment of copper deficiency: Relation to mitochondrial turnover. Journal of Clinical Investigation, 46, 1819.
Bereiter-Hahn, J. (1990). Behavior of mitochondria in the living cell. International Review of Cytology, 122, 1–63.
Skulachev, V. P. (2001). Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends in Biochemical Sciences, 26, 23–29.
Twig, G., Elorza, A., Molina, A. J. A., Mohamed, H., Wikstrom, J. D., Walzer, G., et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO Journal, 27, 433–446.
Santel, A., & Fuller, M. T. (2001). Control of mitochondrial morphology by a human mitofusin. Journal of Cell Science, 114, 867–874.
Smirnova, E., Shurland, D.-L., Ryazantsev, S. N., & van der Bliek, A. M. (1998). A human dynamin-related protein controls the distribution of mitochondria. The Journal of Cell Biology, 143, 351–358.
Hoffmann, P., Richards, D., Heinroth-Hoffmann, I., Mathias, P., Wey, H., & Toraason, M. (1995). Arachidonic acid disrupts calcium dynamics in neonatal rat cardiac myocytes. Cardiovascular Research, 30, 889–898.
Wang, T., Li, R., Lin, C., Sun, M., & Kang, Y. J. (2014). Brief communication: Copper suppression of vascular endothelial growth factor receptor-2 is involved in the regression of cardiomyocyte hypertrophy. Experimental Biology and Medicine, 239, 948–953.
Zhou, Y., Jiang, Y., & Kang, Y. J. (2008). Copper reverses cardiomyocyte hypertrophy through vascular endothelial growth factor-mediated reduction in the cell size. Journal of Molecular and Cellular Cardiology, 45, 106–117.
Schaper, J., Froede, R., Hein, S. T., Buck, A., Hashizume, H., Speiser, B., et al. (1991). Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation, 83, 504–514.
Zak, R., Rabinowitz, M., Rajamanickam, C., Merten, S., & Kwiatkowska-Patzer, B. (1980). Mitochondrial proliferation in cardiac hypertrophy. Basic Research in Cardiology, 75, 171–178.
Duvezin-Caubet, S., Jagasia, R., Wagener, J., Hofmann, S., Trifunovic, A., Hansson, A., et al. (2006). Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. Journal of Biological Chemistry, 281, 37972–37979.
Baandrup, U., Florio, R. A., Roters, F., & Olsen, E. G. (1981). Electron microscopic investigation of endomyocardial biopsy samples in hypertrophy and cardiomyopathy. A semiquantitative study in 48 patients. Circulation, 63, 1289–1298.
Pennanen, C., Parra, V., López-Crisosto, C., Morales, P. E., del Campo, A., Gutierrez, T., et al. (2014). Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. Journal of Cell Science, 127, 2659–2671.
Coronado, M., Fajardo, G., Nguyen, K., Zhao, M., Kooiker, K. B., Hu, D.-Q., et al. (2017). Physiologic mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circulation Research. https://doi.org/10.1161/CIRCRESAHA.1117.310725.
Medeiros, D. M., Bagby, D., Ovecka, G., & McCormick, R. (1991). Myofibrillar, mitochondrial and valvular morphological alterations in cardiac hypertrophy among copper-deficient rats. The Journal of Nutrition, 121, 815–824.
Papanicolaou, K. N., Ngoh, G. A., Dabkowski, E. R., O’Connell, K. A., Ribeiro, R. F., Jr., Stanley, W. C., et al. (2011). Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. American Journal of Physiology-Heart and Circulatory Physiology, 302, H167–H179.
Cereghetti, G. M., Stangherlin, A., De Brito, O. M., Chang, C. R., Blackstone, C., Bernardi, P., et al. (2008). Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proceedings of the National Academy of Sciences, 105, 15803–15808.
Jhun, B. S., Adaniya, S. M., Mancini, T. J., Cao, J. L., King, M. E., Landi, A. K., et al. (2018). Protein kinase D activation induces mitochondrial fragmentation and dysfunction in cardiomyocytes. The Journal of Physiology. https://doi.org/10.1113/JP275418.
Fang, L., Moore, X.-L., Gao, X.-M., Dart, A. M., Lim, Y. L., & Du, X.-J. (2007). Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sciences, 80, 2154–2160.
Chen, L., Gong, Q., Stice, J. P., & Knowlton, A. A. (2009). Mitochondrial OPA1, apoptosis, and heart failure. Cardiovascular Research, 84, 91–99.
Sun, M., Zuo, X., Li, R., Wang, T., & Kang, Y. J. (2014). Vascular endothelial growth factor recovers suppressed cytochrome c oxidase activity by restoring copper availability in hypertrophic cardiomyocytes. Experimental Biology and Medicine, 239, 1671–1677.
Zuo, X., Dong, D., Sun, M., Xie, H., & Kang, Y. J. (2013). Homocysteine restricts copper availability leading to suppression of cytochrome C oxidase activity in phenylephrine-treated cardiomyocytes. PLoS ONE, 8, e67549.
Radford, N. B., Wan, B., Richman, A., Szczepaniak, L. S., Li, J.-L., Li, K., et al. (2002). Cardiac dysfunction in mice lacking cytochrome-c oxidase subunit VIaH. American Journal of Physiology-Heart and Circulatory Physiology, 282, H726–H733.
Acknowledgements
The authors want to thank Lin Bai, Suzheng Fan for providing technical assistance for mitochondrial imaging and AAS, respectively. This study was supported by National Science Foundation of China (Grant Number 81230004) and Sichuan University West China Hospital.
Author information
Authors and Affiliations
Contributions
All authors participated in the design, analysis of the data, interpretation of the results, and review of the manuscript; WY, RL, and XRF carried out the experiments; YJK and WY wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Yin, W., Li, R., Feng, X. et al. The Involvement of Cytochrome c Oxidase in Mitochondrial Fusion in Primary Cultures of Neonatal Rat Cardiomyocytes. Cardiovasc Toxicol 18, 365–373 (2018). https://doi.org/10.1007/s12012-018-9447-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-9447-1