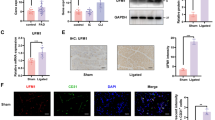
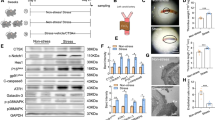
Abstract
SSeCKS/Gravin/AKAP12 is a protein kinase C (PKC) substrate that inhibits the activity of PKC through binding with it. SSeCKS is expressed in vascular endothelial cells (ECs). The atypical PKC isoform ζ (PKCζ) is a pathologic mediator of endothelial dysfunction. However, the functional significance of SSeCKS/PKCζ dimerization in the vascular endothelium remains poorly understood. Given this background, we investigated the effects of SSeCKS on endothelial dysfunction and elucidated the possible mechanism involved. Vascular endothelial dysfunction and inflammatory changes were induced by treatment with bacterial endotoxin lipopolysaccharide (LPS, a vascular endothelial toxicity inducer). LPS can increase the level of SSeCKS. However, we also found that depletion of SSeCKS aggravated the LPS-induced vascular endothelial dysfunction, upregulated pro-inflammatory proteins and phosphorylation level of PKCζ, increased ROS formation, decreased extracellular-signal-regulated kinase 5 (ERK5) transcriptional activity, and reduced eNOS expression. Further examination revealed that depletion of SSeCKS increased PKCζ/ERK5 dimerization. These findings provide preliminary evidence that the expression of SSeCKS induced by LPS, as a negative feedback mechanism, has the potential to improve endothelium-dependent relaxation in vascular disease conditions by inhibiting PKCζ-mediated reduction of ERK5 transactivation.




Similar content being viewed by others
Abbreviations
- PKCζ:
-
Protein kinases C isoformsζ
- ECs:
-
Vascular endothelial cells
- LPS:
-
Lipopolysaccharide
- ERK5:
-
Extracellular-signal-regulated kinase 5
- ACh:
-
Acetylcholine
References
Lin, X., Nelson, P., & Gelman, I. H. (2000). SSeCKS, a major protein kinase C substrate with tumor suppressor activity, regulates G(1)-->S progression by controlling the expression and cellular compartmentalization of cyclin D. Molecular and Cellular Biology, 20, 7259–7272.
Guo, L. W., Gao, L., Rothschild, J., Su, B., & Gelman, I. H. (2011). Control of protein kinase C activity, phorbol ester-induced cytoskeletal remodeling, and cell survival signals by the scaffolding protein SSeCKS/GRAVIN/AKAP12. Journal of Biological Chemistry, 286, 38356–38366.
Weissmuller, T., Glover, L. E., Fennimore, B., Curtis, V. F., MacManus, C. F., Ehrentraut, S. F., et al. (2014). HIF-dependent regulation of AKAP12 (gravin) in the control of human vascular endothelial function. FASEB Journal, 28, 256–264.
Yan, M., Zhao, J., Zhu, S., Shao, X., Zhang, L., Gao, H., et al. (2014). Expression of SRC suppressed C kinase substrate in rat neural tissues during inflammation. Neurochemical Research, 39, 748–757.
Cheng, C., Liu, H., Ge, H., Qian, J., Qin, J., Sun, L., et al. (2007). Lipopolysaccharide induces expression of SSeCKS in rat lung microvascular endothelial cell. Molecular and Cellular Biochemistry, 305, 1–8.
Yang, Z., Breider, M. A., Carroll, R. C., Miller, M. S., & Bochsler, P. N. (1996). Soluble CD14 and lipopolysaccharide-binding protein from bovine serum enable bacterial lipopolysaccharide-mediated cytotoxicity and activation of bovine vascular endothelial cells in vitro. Journal of Leukocyte Biology, 59, 241–247.
Akakura, S., Nochajski, P., Gao, L., Sotomayor, P., Matsui, S., & Gelman, I. H. (2010). Rb-dependent cellular senescence, multinucleation and susceptibility to oncogenic transformation through PKC scaffolding by SSeCKS/AKAP12. Cell Cycle, 9, 4656–4665.
Song, H. B., Jun, H. O., Kim, J. H., Yu, Y. S., Kim, K. W., & Kim, J. H. (2014). Suppression of protein kinase C-zeta attenuates vascular leakage via prevention of tight junction protein decrease in diabetic retinopathy. Biochemical and Biophysical Research Communications, 444, 63–68.
Li, Z. L., Liu, J. C., Liu, S. B., Li, X. Q., Yi, D. H., & Zhao, M. G. (2012). Improvement of vascular function by acute and chronic treatment with the GPR30 agonist G1 in experimental diabetes mellitus. PLoS ONE, 7, e38787.
Hecquet, C. M., Ahmmed, G. U., & Malik, A. B. (2010). TRPM2 channel regulates endothelial barrier function. Advances in Experimental Medicine and Biology, 661, 155–167.
Sturza, A., Leisegang, M. S., Babelova, A., Schroder, K., Benkhoff, S., Loot, A. E., et al. (2013). Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension, 62, 140–146.
Lund, D. D., Brooks, R. M., Faraci, F. M., & Heistad, D. D. (2007). Role of angiotensin II in endothelial dysfunction induced by lipopolysaccharide in mice. American Journal of Physiology Heart Circulatory Physiology, 293, H3726–H3731.
Witzenbichler, B., Westermann, D., Knueppel, S., Schultheiss, H. P., & Tschope, C. (2005). Protective role of angiopoietin-1 in endotoxic shock. Circulation, 111, 97–105.
Lu, J. L., Schmiege, L. M. 3rd, Kuo, L., & Liao, J. C. (1996). Downregulation of endothelial constitutive nitric oxide synthase expression by lipopolysaccharide. Biochemical and Biophysical Research Communications, 225, 1–5.
Yazji, I., Sodhi, C. P., Lee, E. K., Good, M., Egan, C. E., Afrazi, A., et al. (2013). Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proceedings of the National Academy of Sciences USA, 110, 9451–9456.
Jonigk, D., Al-Omari, M., Maegel, L., Muller, M., Izykowski, N., Hong, J., et al. (2013). Anti-inflammatory and immunomodulatory properties of alpha1-antitrypsin without inhibition of elastase. Proceedings of the National Academy of Sciences USA, 110, 15007–15012.
Westerterp, M., Berbee, J. F., Pires, N. M., van Mierlo, G. J., Kleemann, R., Romijn, J. A., et al. (2007). Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation, 116, 2173–2181.
Liang, C. F., Liu, J. T., Wang, Y., Xu, A., & Vanhoutte, P. M. (2013). Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arteriosclerosis Thrombosis and Vascular Biology, 33, 777–784.
Libby, P. (2002). Inflammation in atherosclerosis. Nature, 420, 868–874.
Park, J. H., Jeong, Y. J., Won, H. K., Choi, S. Y., Park, J. H., & Oh, S. M. (2014). Activation of TOPK by lipopolysaccharide promotes induction of inducible nitric oxide synthase through NF-kappaB activity in leukemia cells. Cellular Signalling, 26, 849–856.
Capiralla, H., Vingtdeux, V., Venkatesh, J., Dreses-Werringloer, U., Zhao, H., Davies, P., et al. (2012). Identification of potent small-molecule inhibitors of STAT3 with anti-inflammatory properties in RAW 264.7 macrophages. FEBS Journal, 279, 3791–3799.
Nigro, P., Abe, J., Woo, C. H., Satoh, K., McClain, C., O’Dell, M. R., et al. (2010). PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood, 116, 1971–1979.
Nithianandarajah-Jones, G. N., Wilm, B., Goldring, C. E., Muller, J., & Cross, M. J. (2012). ERK5: structure, regulation and function. Cellular Signalling, 24, 2187–2196.
Dong, F., Gutkind, J. S., & Larner, A. C. (2001). Granulocyte colony-stimulating factor induces ERK5 activation, which is differentially regulated by protein-tyrosine kinases and protein kinase C. Regulation of cell proliferation and survival. Journal of Biological Chemistry, 276, 10811–10816.
Sohn, S. J., Sarvis, B. K., Cado, D., & Winoto, A. (2002). ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. Journal of Biological Chemistry, 277, 43344–43351.
Regan, C. P., Li, W., Boucher, D. M., Spatz, S., Su, M. S., & Kuida, K. (2002). Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proceedings of the National Academy of Sciences USA, 99, 9248–9253.
Parmar, K. M., Larman, H. B., Dai, G., Zhang, Y., Wang, E. T., Moorthy, S. N., et al. (2006). Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. Journal of Clinical Investigation, 116, 49–58.
Dekker, R. J., van Soest, S., Fontijn, R. D., Salamanca, S., de Groot, P. G., VanBavel, E., et al. (2002). Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood, 100, 1689–1698.
SenBanerjee, S., Lin, Z., Atkins, G. B., Greif, D. M., Rao, R. M., Kumar, A., et al. (2004). KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. Journal of Experimental Medicine, 199, 1305–1315.
Wu, K., Tian, S., Zhou, H., & Wu, Y. (2013). Statins protect human endothelial cells from TNF-induced inflammation via ERK5 activation. Biochemical Pharmacology, 85, 1753–1760.
Chen, L., Liu, J. C., Zhang, X. N., Guo, Y. Y., Xu, Z. H., Cao, W., et al. (2008). Down-regulation of NR2B receptors partially contributes to analgesic effects of Gentiopicroside in persistent inflammatory pain. Neuropharmacology, 54, 1175–1181.
Reinhart-King, C. A., Fujiwara, K., & Berk, B. C. (2008). Physiologic stress-mediated signaling in the endothelium. Methods in Enzymology, 443, 25–44.
Bagi, Z., Frangos, J. A., Yeh, J. C., White, C. R., Kaley, G., & Koller, A. (2005). PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arteriosclerosis Thrombosis and Vascular Biology, 25, 1590–1595.
Kumagai, R., Lu, X., & Kassab, G. S. (2009). Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Radical Biology and Medicine, 47, 600–607.
Hou, S., Ding, H., Lv, Q., Yin, X., Song, J., Landen, N. X., et al. (2014). Therapeutic effect of intravenous infusion of perfluorocarbon emulsion on LPS-induced acute lung injury in rats. PLoS ONE, 9, e87826.
Yang, N., Liu, Y. Y., Pan, C. S., Sun, K., Wei, X. H., Mao, X. W., et al. (2014). Pre-treatment with andrographolide pills attenuates lipopolysaccharide-induced pulmonary microcirculatory disturbance and acute lung injury in rats. Microcirculation, 21, 703–716
Lee, K. S., Kim, J., Kwak, S. N., Lee, K. S., Lee, D. K., Ha, K. S., et al. (2014). Functional role of NF-kappaB in expression of human endothelial nitric oxide synthase. Biochemical and Biophysical Research Communications, 448, 101–107.
Woo, C. H., Shishido, T., McClain, C., Lim, J. H., Li, J. D., Yang, J., et al. (2008). Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circulation Research, 102, 538–545.
Surapisitchat, J., Hoefen, R. J., Pi, X., Yoshizumi, M., Yan, C., & Berk, B. C. (2001). Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proceedings of the National Academy of Sciences USA, 98, 6476–6481.
Collins, A. R., Meehan, W. P., Kintscher, U., Jackson, S., Wakino, S., Noh, G., et al. (2001). Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arteriosclerosis Thrombosis and Vascular Biology, 21, 365–371.
Akaike, M., Che, W., Marmarosh, N. L., Ohta, S., Osawa, M., Ding, B., et al. (2004). The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Molecular and Cellular Biology, 24, 8691–8704.
Leverence, J. T., Medhora, M., Konduri, G. G., & Sampath, V. (2011). Lipopolysaccharide-induced cytokine expression in alveolar epithelial cells: role of PKCzeta-mediated p47phox phosphorylation. Chemico Biological Interactions, 189, 72–81.
Acknowledgements
Jian Zuo was supported by National Key R&D Plan of China (Grant No. 2016YFC1301901). Zilin Li was supported by National Natural Science Foundation of China (Grant No. 81400276). Jing Hu was supported by PLA medical science and Technology Youth cultivation project (Grant No. 14QNP018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest has been declared.
Additional information
Handling Editor: Mitzi C. Glover.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

12012_2018_9502_MOESM1_ESM.jpg
Supplementary Figure 1 The expressions of SSeCKS protein in HUVECs. (A) SSeCKS expression after 48-hour transfection with SSeCKS shRNA lentivirus particles in HUVECs. (red = SSeCKS). (B) Western Blot analysis of SSeCKS expression showed that transfection with SSeCKS shRNA lentivirus particles showed reduction in SSeCKS expression compared to control. **p < 0.01. Results are given as the mean ± SEM of three independent experiments. (JPG 366 KB)

12012_2018_9502_MOESM2_ESM.jpg
Supplementary Figure 2 (A) HUVECs were transfected with control or SSeCKS shRNAs for 48 hrs and then treated with or without LPS for 6 h. SSeCKS expression was increased in HUVECs treated with LPS as compared with those treated without LPS. (B) HUVECs were transduced with either adenovirus vector containing Lacz (Ad-Lacz) or SSeCKS (Ad-SSeCKS). After 24h of transduction, cells were treated with or without LPS (30ng/ml) and then exposed to s-flow for 24 h. NO production was decreased in HUVECs treated with LPS as compared with those untreated with LPS. SSeCKS reversed LPS-mediated downregulation of NO production. *p < 0.05, **p < 0.01. Results are given as the mean ± SEM of three independent experiments. (JPG 633 KB)

12012_2018_9502_MOESM3_ESM.jpg
Supplementary Figure 3 Effect of SSeCKS on the relaxation response to SNP. The capability of the relaxation caused by SNP (10-9 M to 10-5 M) had no difference in the aortic segments among the four groups. n = 6–12 rings from 5–8 rats. **p <0.01. Results are given as the mean ± SEM of three independent experiments. (JPG 117 KB)

12012_2018_9502_MOESM4_ESM.jpg
Supplementary Figure 4 Working model of the SSeCKS signaling pathway inhibiting PKCζ-mediated reduction of ERK5 transactivation to prevent endothelial dysfunction. (JPG 180 KB)

12012_2018_9502_MOESM5_ESM.jpg
Supplementary Figure 5 NF-kB activity assay. HUVECs were transfected with control or SSeCKS shRNAs for 48 h and then treated with or without LPS for 6 h. Nuclear protein extracts were harvested and NF-κB activity was measured by transAM NFκB p65 protein kit. *p < 0.05. Results are given as the mean ± SEM of three independent experiments. (JPG 123 KB)

12012_2018_9502_MOESM6_ESM.jpg
Supplementary Figure 6 The maximum constriction of PE of the aortic rings from the LPS group is lower than the aortic rings from control group (**p <0.01, LPS group vs. control group). But, no significant difference was found between the LPS group and LPS+shRNA-SSeCKS group. n = 7–15 rings from 6–9 rats. **p <0.01. Results are given as the mean ± SEM of three independent experiments. (JPG 139 KB)
Rights and permissions
About this article
Cite this article
Li, Z., Hu, J., Guo, J. et al. SSeCKS/Gravin/AKAP12 Inhibits PKCζ-Mediated Reduction of ERK5 Transactivation to Prevent Endotoxin-Induced Vascular dysfunction. Cardiovasc Toxicol 19, 372–381 (2019). https://doi.org/10.1007/s12012-018-09502-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-09502-9