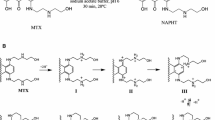
Abstract
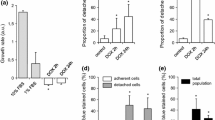
Mitoxantrone (MTX) is a chemotherapeutic agent that emerged as an alternative to anthracycline therapy. However, MTX also causes late cardiotoxicity, being oxidative stress and mitochondrial-impaired function proposed as possible mechanisms. This work aimed to investigate the relevance of these mechanisms to the MTX toxicity in H9c2 cells, using therapeutic concentrations. The observed cytotoxicity of MTX was time and concentration dependent in both lactate dehydrogenase leakage assay and MTT reduction assay. Two therapeutic concentrations (100 nM and 1 μM) and three time points were selected (24, 48, and 96 h) for further studies. Both MTX concentrations caused a significant increase in caspase-3 activity, which was not prevented by inhibiting MTX CYP450-metabolism. Significant decreases were observed in the total and reduced glutathione levels only in MTX 100 nM at 96 h; however, neither alterations in oxidized glutathione nor increases in the malondialdehyde levels were observed at any time or concentrations tested. On the other hand, changes in the intracellular ATP levels, mitochondrial membrane potential, and intracellular calcium levels were observed in both concentrations and all time tested. Noteworthy, decreased levels of ATP-synthase expression and activity and increases in the reactive species generation were observed at 96 h in both working concentrations. However, the radical scavenger N-acetylcysteine or the mitochondrial function enhancer L-carnitine did not prevent MTX cytotoxicity. Thus, this work evidenced the early MTX-induced energetic crisis as a possible key factor in the cell injury.







Similar content being viewed by others
Abbreviations
- DCFH-DA:
-
Dichlorodihydrofluorescein diacetate
- DHR:
-
Dihydrorhodamine 123
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- DTNB:
-
5,5-Dithio-bis(2-nitrobenzoic) acid
- FBS:
-
Fetal bovine serum
- FI:
-
Fluorescence intensity
- GSH:
-
Reduced glutathione
- GSHt:
-
Total glutathione
- i.p:
-
Intraperitoneal
- GSSG:
-
Oxidized glutathione
- LDH:
-
Lactate dehydrogenase
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MTX:
-
Mitoxantrone
- NAC:
-
N-acetylcysteine
- β-NADH:
-
Reduced β-nicotinamide adenine dinucleotide
- Pi:
-
Inorganic phosphate
- SD:
-
Standard deviation
- TBA:
-
Thiobarbituric acid
- TMRM:
-
Tetramethylrhodamine
References
Seiter, K. (2005). Toxicity of the topoisomerase II inhibitors. Expert Opinion on Drug Safety, 4, 219–234.
Kingwell, E., Koch, M., Leung, B., Isserow, S., Geddes, J., Rieckmann, P., et al. (2010). Cardiotoxicity and other adverse events associated with mitoxantrone treatment for MS. Neurology, 74, 1822–1826.
Khan, S. N., Lai, S. K., Kumar, P., & Khan, A. U. (2010). Effect of mitoxantrone on proliferation dynamics and cell cycle progression. Bioscience Reports, 30, 375–381.
Hajihassan, Z., & Rabbani-Chadegani, A. (2011). Interaction of mitoxantrone, as an anticancer drug, with chromatin proteins, core histones and H1, in solution. International Journal of Biological Macromolecules, 48, 87–92.
Alderton, P. M., Gross, J., & Green, M. D. (1992). Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Research, 52, 194–201.
Batra, V. K., Morrison, J. A., Woodward, D. L., Siverd, N. S., & Yacobi, A. (1986). Pharmacokinetics of mitoxantrone in man and laboratory animals. Drug Metabolism Reviews, 17, 311–329.
Sereno, M., Brunello, A., Chiappori, A., Barriuso, J., Casado, E., Belda, C., et al. (2008). Cardiac toxicity: Old and new issues in anti-cancer drugs. Clinical and Translational Oncology, 10, 35–46.
Wallace, K. B. (2003). Doxorubicin-induced cardiac mitochondrionopathy. Pharmacology and Toxicology, 93, 105–115.
Brück, T. B., & Brück, D. W. (2011). Oxidative metabolism of the anti-cancer agent mitoxantrone by horseradish, lacto-and lignin peroxidase. Biochimie, 93, 217–226.
Duthie, S. J., & Grant, M. H. (1989). The role of reductive and oxidative metabolism in the toxicity of mitoxantrone, adriamycin and menadione in human liver derived Hep G2 hepatoma cells. British Journal of Cancer, 60, 566–571.
Panousis, C., Kettle, A., & Phillips, D. R. (1995). Myeloperoxidase oxidises mitoxantrone to metabolites which bind covalently DNA and RNA. Anticancer drug designer, 10, 593–605.
Mewes, K., Blanz, J., Ehninger, G., Gebhardt, R., & Zeller, K. P. (1993). Cytochrome P-450-induced cytotoxicity of mitoxantrone by formation of electrophilic intermediates. Cancer Research, 53, 5135–5142.
Li, S. J., Rodgers, E. H., & Grant, M. H. (1995). The activity of xenobiotic enzymes and the cytotoxicity of mitoxantrone in MCF 7 human breast cancer cells treated with inducing agents. Chemico Biological Interactions, 97, 101–118.
Rossato, L., Costa, V., De Pinho, P., Freitas, V., Viloune, L., Bastos, M., et al. (2013). The metabolic profile of mitoxantrone and its relation with mitoxantrone-induced cardiotoxicity. Archives of Toxicology. doi:10.1007/s00204-013-1040-6.
Shipp, N. G., Dorr, R. T., Alberts, D. S., Dawson, B. V., & Hendrix, M. (1993). Characterization of experimental mitoxantrone cardiotoxicity and its partial inhibition by ICRF-187 in cultured neonatal rat heart cells. Cancer Research, 53, 550–556.
Bachmann, E., Weber, E., & Zbinden, G. (1987). Effect of mitoxantrone and doxorubicin on energy metabolism of the rat heart. Cancer Treatment Reports, 71, 361–366.
Kimes, B. W., & Brandt, B. L. (1976). Properties of a clonal muscle from rat heart. Experimental Cell Research, 98, 367–381.
Zordoky, B. N. M., & El-Kadi, A. O. S. (2007). H9c2 cell line is a valuable in vitro model to study the drug metabolizing enzymes in the heart. Journal of Pharmacological and Toxicological Methods, 56, 317–322.
Costa, V. M., Silva, R., Ferreira, R., Amado, F., Carvalho, F., Bastos, M. L., et al. (2009). Adrenaline in pro-oxidant conditions elicits intracellular survival pathways in isolated rat cardiomyocytes. Toxicology, 257, 70–79.
Rossato, L. G., Costa, V. M., De Pinho, P. G., Carvalho, F., Bastos, M. L., & Remião, F. (2011). Structural isomerization of synephrine influences its uptake and ensuing glutathione depletion in rat-isolated cardiomyocytes. Archives of Toxicology, 85, 929–939.
Sayed-Ahmed, M. M., Shaarawy, S., Shouman, S. A., & Osman, A. M. (1999). Reversal of doxorubicin-induced cardiac metabolic damage by L-carnitine. Pharmacological Research, 39, 289–295.
Angeloni, C., Spencer, J. P. E., Leoncini, E., Biagi, P. L., & Hrelia, S. (2007). Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie, 89, 73–82.
Grotto, D., Santa Maria, L. D., Boeira, S., Valentini, J., Charão, M. F., Moro, A. M., et al. (2007). Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. Journal of Pharmaceutical and Biomedical Analysis, 43, 619–624.
Costa, V. M., Silva, R., Ferreira, L. M., Branco, P. S., Carvalho, F., Bastos, M. L., et al. (2007). Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: Formation of adrenochrome, quinoproteins, and GSH adduct. Chemical Research in Toxicology, 20, 1183–1191.
Floryk, D., & Houstĕk, J. (1999). Tetramethyl rhodamine methyl ester (TMRM) is suitable for cytofluorometric measurements of mitochondrial membrane potential in cells treated with digitonin. Bioscience Reports, 19, 27–34.
Carvalho, M., Remião, F., Milhazes, N., Borges, F., Fernandes, E., Monteiro, M. D. C., et al. (2004). Metabolism is required for the expression of ecstasy-induced cardiotoxicity in vitro. Chemical Research in Toxicology, 17, 623–632.
Canal, P., Attal, M., Chatelut, E., Guichard, S., Huguet, F., Muller, C., et al. (1993). Plasma and cellular pharmacokinetics of mitoxantrone in high-dose chemotherapeutic regimen for refractory lymphomas. Cancer Research, 53, 4850–4854.
Neuhaus, O., Kieseier, B. C., & Hartung, H.-P. (2006). Therapeutic role of mitoxantrone in multiple sclerosis. Pharmacology & Therapeutics, 109, 198–209.
Kluza, J., Marchetti, P., Gallego, M.-A., Lancel, S., Fournier, C., Loyens, A., et al. (2004). Mitochondrial proliferation during apoptosis induced by anticancer agents: Effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene, 23, 7018–7030.
Fisher, G. R., & Patterson, L. H. (1992). Lack of involvement of reactive oxygen in the cytotoxicity of mitoxantrone, CI941 and ametantrone in MCF-7 cells: comparison with doxorubicin. Cancer Chemotherapy and Pharmacology, 30, 451–458.
Chen, F., Lewis, W., Hollander, J. M., Baseler, W., & Finkel, M. S. (2012). N-acetylcysteine reverses cardiac myocyte dysfunction in HIV-Tat proteinopathy. Journal of Applied Physiology, 113, 105–113.
Chen, F., Hadfield, J., Berzingi, C., Hollander, J., Miller, D., Nichols, C., et al. (2013). N-acetylcysteine reverses cardiac myocyte dysfunction in a rodent model of behavioral stress. Journal of Applied Physiology, 115, 514–524.
Arnaiz, S. L., & Llesuy, S. (1993). Oxidative stress in mouse heart by antitumoral drugs: A comparative study of doxorubicin and mitoxantrone. Toxicology, 77, 31–38.
Novak, R. F., & Kharasch, E. D. (1985). Mitoxantrone: Propensity for free radical formation and lipid peroxidation-implications for cardiotoxicity. Investigational New Drugs, 3, 95–99.
Dong, Z., Saikumar, P., Weinberg, J. M., & Venkatachalam, M. A. (2006). Calcium in cell injury and death. Annual Review of Pathology: Mechanisms of Disease, 1, 405–434.
Li, R., Beebe, T., Cui, J., Rouhanizadeh, M., Ai, L., Wang, P., et al. (2009). Pulsatile shear stress increased mitochondrial membrane potential: Implication of Mn-SOD. Biochemical and Biophysical Research Communications, 388, 406–412.
Acton, B. M. (2004). Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Molecular Human Reproduction, 10, 23–32.
Zammit, V. A., Ramsay, R. R., Bonomini, M., & Arduini, A. (2009). Carnitine, mitochondrial function and therapy. Advanced Drug Delivery Reviews, 61, 1353–1362.
Mijares, A., & López, J. R. (2001). L-carnitine prevents increase in diastolic [Ca2+] induced by doxorubicin in cardiac cells. European Journal of Pharmacology, 425, 117–120.
Newman, R., Hacker, M., & Krakoff, I. (1981). Amelioration of adriamycin and daunorubicin myocardial toxicity by adenosine. Cancer Research, 41, 3483–3488.
Costa, V. M., Carvalho, F., Bastos, M. L., Carvalho, R. A., Carvalho, M., & Remião, F. (2011). Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Current Medical Chemistry, 18, 2272–2314.
Trump, B. F., & Berezesky, I. K. (1995). Calcium-mediated cell injury and cell death. FASEB, 9, 219–228.
Acknowledgments
This work received financial support from “Fundação para a Ciência e Tecnologia (FCT),” Portugal (EXPL/DTP-FTO/0290/2012) and by “Fundo Comunitário Europeu” (FEDER) under the frame of “Eixo I do Programa Operacional Fatores de Competitividade (POFC) do QREN” (COMPETE: FCOMP-01-0124-FEDER-027749). The work was also supported by FCT within the framework of Strategic Projects for Scientific Research Units of R&D (project PEst-C/EQB/LA0006/2011). LGR and VVB thank FCT for their PhD Grant (SFRH/BD/63473/2009 and SFRH/BD/82556/2011, respectively) and VMC thank FCT for her Post-doc Grant (SFRH/BPD/63746/2009). Authors are grateful to Dr. Vilma Sardão, from University of Coimbra, for gently providing us with the cellular model used in this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rossato, L.G., Costa, V.M., Vilas-Boas, V. et al. Therapeutic Concentrations of Mitoxantrone Elicit Energetic Imbalance in H9c2 Cells as an Earlier Event. Cardiovasc Toxicol 13, 413–425 (2013). https://doi.org/10.1007/s12012-013-9224-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9224-0