Abstract
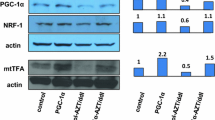
Despite the highly effective impact of NRTI therapy in patients infected with the human immunodeficiency virus type 1 (HIV-1), long-term treatment has revealed cardiotoxicity, considered to be due to mitochondrial dysfunction. To evaluate mitochondrial damage, and design therapeutic interventions, we established cultures of rat H9c2 and mouse HL-1 cardiomyocytes and exposed them to the NRTIs zidovudine (AZT), and AZT plus didanosine (ddI). Proliferation assays showed that H9c2 cells grew well in 50 μM AZT and 50 μM AZT/50 μM ddI and that HL-1 cells grew well in 10 μM AZT and 10 μM AZT/10 μM ddI. Both types of cells were exposed to the drugs for 39 passages (P), and mitochondrial integrity in the form of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) was examined by Seahorse XF24 analyzer. In NRTI-exposed H9c2 cells at most passages, OCR was reduced, in both the basal and uncoupled states, compared to unexposed controls (P < 0.05). NRTI-exposed HL-1 cells showed a different pattern of mitochondrial compromise, with inhibition of OCR, in basal and uncoupled cells, occurring largely before P14 and after P17 (P < 0.05). The ECAR response in uncoupled cells of both types was unchanged at early passages, but increased after P18 (P < 0.05). Evaluation of mitochondrial biogenesis in H9c2 cells revealed reduction before P29, no change at P29, and reduction at P39 in NRTI-exposed cells, compared to unexposed cells (P < 0.05). Western blotting of transcription factors critical for mitochondrial biogenesis, PGC-1α, Nrf-1 and mtTFA, showed downregulation in NRTI-exposed H9c2 cells compared to unexposed controls. In addition, electron microscopy (EM) revealed increasing mitochondrial morphological damage in H9c2 cells over passages. For both cell types, AZT/ddI was more damaging than AZT alone. These studies demonstrate progressive mitochondrial compromise in cardiomyocytes-exposed long term, and the model will be used to evaluate potentially protective intervention strategies.







Similar content being viewed by others
Abbreviations
- HIV-1:
-
Human immunodeficiency virus type 1
- ARV:
-
Antiretroviral
- ATP:
-
Adenosine-5′-triphosphate
- AZT:
-
Zidovudine, 3′-azido-3′-deoxythymidine
- ddI:
-
Didanosine, 2′, 3′-dideoxyinosine
- NRTI:
-
Nucleoside reverse transcriptase inhibitor
- FCCP:
-
Carbonyl cyanide p-trifluoromethoxy-phenylhydrazone
- OLIGO:
-
Oligomycin
- ROT:
-
Rotenone
- HL-1:
-
Mouse cardiomyocytes
- H9c2:
-
Rat cardiomyocytes
- OCR:
-
Oxygen consumption rate
- ECAR:
-
Extracellular acidification rate
- EM:
-
Electron microscopy
- COX-1:
-
Cytochrome c oxidase-1
- SDH-A:
-
Succinate dehydrogenase-A
- mtDNA:
-
Mitochondrial DNA
- nDNA:
-
Nuclear DNA
- OXPHOS:
-
Oxidative phosphorylation
References
HIV/AIDS Statistics and Surveillance, CDC. Available from: http://www.cdc.gov/hiv/topics/surveillance/basic.htm. Accessed July 1, 2011.
Condra, J. H., Miller, M. D., Hazuda, D. J., & Emini, E. A. (2002). Potential new therapies for the treatment of HIV-1 infection. Annual Review of Medicine, 53, 541–555.
Connor, E. M., Sperling, R. S., Gelber, R., Kiselev, P., Scott, G., O’Sullivan, M. J., et al. (1994). Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. New England Journal of Medicine, 331, 1173–1180.
Mofenson, L. M. (2004). Successes and challenges in the perinatal HIV-1 epidemic in the United States as illustrated by the HIV-1 Serosurvey of childbearing women. Archives of Pediatrics and Adolescent Medicine, 158, 422–425.
Watts, D. H. (2006). Treating HIV during pregnancy: An update on safety issues. Drug Safety, 29, 467–490.
Lewis, W., Day, B. J., & Copeland, W. C. (2003). Mitochondrial toxicity of NRTI antiviral drugs: An integrated cellular perspective. Nature Reviews Drug Discovery, 2, 812–822.
Moyle, G. (2000). Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clinical Therapeutics, 22, 911–936.
Nolan, D., & Mallal, S. (2004). Complications associated with NRTI therapy: Update on clinical features and possible pathogenic mechanisms. Antiviral Therapy, 9, 849–863.
Poirier, M. C., Olivero, O. A., Walker, D. M., & Walker, V. E. (2004). Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicology and Applied Pharmacology, 199, 151–161.
Carter, M. M., Torres, S. M., Cook, D. L., Jr., McCash, C. L., Yu, M., Walker, V. E., et al. (2007). Relative mutagenic potencies of several nucleoside analogs, alone or in drug pairs, at the HPRT and TK loci of human TK6 lymphoblastoid cells. Environmental and Molecular Mutagenesis, 48, 239–247.
Escobar, P. A., Olivero, O. A., Wade, N. A., Abrams, E. J., Nesel, C. J., Ness, R. B., et al. (2007). Genotoxicity assessed by the comet and GPA assays following in vitro exposure of human lymphoblastoid cells (H9) or perinatal exposure of mother-child pairs to AZT or AZT-3TC. Environmental and Molecular Mutagenesis, 48, 330–343.
Meng, Q., Olivero, O. A., Fasco, M. J., Bellisario, R., Kaminsky, L., Pass, K. A., et al. (2007). Plasma and cellular markers of 3′-azido-3′-dideoxythymidine (AZT) metabolism as indicators of DNA damage in cord blood mononuclear cells from infants receiving prepartum NRTIs. Environmental and Molecular Mutagenesis, 48, 307–321.
Walker, D. M., Kajon, A. E., Torres, S. M., Carter, M. M., McCash, C. L., Swenberg, J. A., et al. (2009). WR1065 mitigates AZT-ddI-induced mutagenesis and inhibits viral replication. Environmental and Molecular Mutagenesis, 50, 460–472.
Borojerdi, J. P., Ming, J., Cooch, C., Ward, Y., Semino-Mora, C., Yu, M., et al. (2009). Centrosomal amplification and aneuploidy induced by the antiretroviral drug AZT in hamster and human cells. Mutation Research, 665, 67–74.
Olivero, O. A., Anderson, L. M., Diwan, B. A., Haines, D. C., Harbaugh, S. W., Moskal, T. J., et al. (1997). Transplacental effects of 3′-azido-2′, 3′-dideoxythymidine (AZT): Tumorigenicity in mice and genotoxicity in mice and monkeys. Journal of the National Cancer Institute, 89, 1602–1608.
Diwan, B. A., Riggs, C. W., Logsdon, D., Haines, D. C., Olivero, O. A., Rice, J. M., et al. (1999). Multiorgan transplacental and neonatal carcinogenicity of 3′-azido-3′-deoxythymidine in mice. Toxicology and Applied Pharmacology, 161, 82–99.
Koczor, C. A., & Lewis, W. (2010). Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opinion on Drug Metabolism & Toxicology, 6, 1493–1504.
Lugassy, D. M., Farmer, B. M., & Nelson, L. S. (2010). Metabolic and hepatobiliary side effects of antiretroviral therapy (ART). Emergency Medicine Clinics of North America, 28, 409–419.
Benhammou, V., Tardieu, M., Warszawski, J., Rustin, P., & Blanche, S. (2007). Clinical mitochondrial dysfunction in uninfected children born to HIV-infected mothers following perinatal exposure to nucleoside analogues. Environmental and Molecular Mutagenesis, 48, 173–178.
Blanche, S., Tardieu, M., Benhammou, V., Warszawski, J., & Rustin, P. (2006). Mitochondrial dysfunction following perinatal exposure to nucleoside analogues. AIDS, 20, 1685–1690.
Blanche, S., Tardieu, M., Rustin, P., Slama, A., Barret, B., Firtion, G., et al. (1999). Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet, 354, 1084–1089.
Meng, Q., Grosovsky, A. J., Shi, X., & Walker, V. E. (2000). Mutagenicity and loss of heterozygosity at the APRT locus in human lymphoblastoid cells exposed to 3′-azido-3′-deoxythymidine. Mutagenesis, 15, 405–410.
Barret, B., Tardieu, M., Rustin, P., Lacroix, C., Chabrol, B., Desguerre, I., et al. (2003). Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: Clinical screening in a large prospective cohort. AIDS, 17, 1769–1785.
Lipshultz, S. E., Shearer, W. T., Thompson, B., Rich, K. C., Cheng, I., Orav, E. J., et al. (2010). Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study). Journal of the American College of Cardiology, 57, 76–85.
Liu, Y., Borchert, G. L., Surazynski, A., & Phang, J. M. (2008). Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene, 27, 6729–6737.
Divi, R. L., Leonard, S. L., Kuo, M. M., Walker, B. L., Orozco, C. C., St Claire, M. C., et al. (2005). Cardiac mitochondrial compromise in 1-yr-old Erythrocebus patas monkeys perinatally-exposed to nucleoside reverse transcriptase inhibitors. Cardiovascular Toxicology, 5, 333–346.
Claycomb, W. C., Lanson, N. A., Jr., Stallworth, B. S., Egeland, D. B., Delcarpio, J. B., Bahinski, A., et al. (1998). HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proceedings of the National Academy Science USA, 95, 2979–2984.
Kimes, B. W., & Brandt, B. L. (1976). Properties of a clonal muscle cell line from rat heart. Experimental Cell Research, 98, 367–381.
Rasbach, K. A., & Schnellmann, R. G. (2007). Signaling of mitochondrial biogenesis following oxidant injury. Journal of Biological Chemistry, 282, 2355–2362.
Liner, K. J., 2nd, Ro, M. J., & Robertson, K. R. (2010). HIV, antiretroviral therapies, and the brain. Current HIV/AIDS Reports, 7, 85–91.
Schouten, J., Cinque, P., Gisslen, M., Reiss, P., & Portegies, P. (2010). HIV-1 infection and cognitive impairment in the cART era: A review. AIDS, 25, 561–575.
Kohler, J. J., & Lewis, W. (2007). A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environmental and Molecular Mutagenesis, 48, 166–172.
Beeson, C. C., Beeson, G. C., & Schnellmann, R. G. (2010). A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Analytical Biochemistry, 404, 75–81.
Arnaudo, E., Dalakas, M., Shanske, S., Moraes, C. T., DiMauro, S., & Schon, E. A. (1991). Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet, 337, 508–510.
Scarpulla, R. C. (2008). Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiological Reviews, 88, 611–638.
Rowe, G. C., Jiang, A., & Arany, Z. (2010). PGC-1 coactivators in cardiac development and disease. Circulation Research, 107, 825–838.
Schon, E. A., DiMauro, S., Hirano, M., & Gilkerson, R. W. (2010). Therapeutic prospects for mitochondrial disease. Trends in Molecular Medicine, 16, 268–276.
Lee, H. C., & Wei, Y. H. (2005). Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. International Journal of Biochemistry and Cell Biology, 37, 822–834.
Olivero, O. A., Fernandez, J. J., Antiochos, B. B., Wagner, J. L., St Claire, M. E., & Poirier, M. C. (2002). Transplacental genotoxicity of combined antiretroviral nucleoside analogue therapy in Erythrocebus patas monkeys. Journal of Acquired Immune Deficiency Syndromes, 29, 323–329.
Torres, S. M., Walker, D. M., Carter, M. M., Cook, D. L., McCash, C. L., Cordova, E. M., et al. (2007). Mutagenicity of zidovudine, lamivudine, and abacavir following in vitro exposure of human lymphoblastoid cells or in utero exposure of CD-1 mice to single agents or drug combinations. Environmental and Molecular Mutagenesis, 48(3–4), 224–238.
Acknowledgments
We wish to thank Dr. Aleksandra Michalowski for statistical consultation. We would also like to thank Dr. Kevin Bittman and Tony Nardei of Seahorse Bioscience Inc. for their help. This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Nguyen, P., Baris, T.Z. et al. Molecular Analysis of Mitochondrial Compromise in Rodent Cardiomyocytes Exposed Long Term to Nucleoside Reverse Transcriptase Inhibitors (NRTIs). Cardiovasc Toxicol 12, 123–134 (2012). https://doi.org/10.1007/s12012-011-9148-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9148-5