Abstract
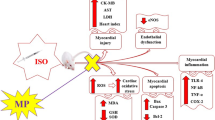
Our previous study described the cardioprotective effects of ellagic acid in an isoproterenol-induced myocardial infarction model. In this study, we are reporting the mechanism of protective action of ellagic acid with respect to apoptosis and mitochondrial respiratory enzymes. Ellagic acid (7.5 and 15 mg/kg) was administered orally as a pretreatment for 10 days. Then, isoproterenol (100 mg/kg) was injected subcutaneously to rats at an interval of 24 h for 2 days. Myocardial infarction was quantified by planimetry. Apoptosis was measured by apoptotic gene expressions. The levels of mitochondrial respiratory enzymes were also measured. Isoproterenol-induced myocardial infarcted rats showed increased infarct size, a decrease in myocardial expression of the Bcl-2 gene and an increase in myocardial expression of the BAX gene. Fas ligand and caspases were markedly elevated along with compromised respiratory marker enzymes in isoproterenol-induced rats. Ellagic acid pretreatment reduced the infarct size, regulated apoptotic gene expressions and enhanced the activities of mitochondrial respiratory marker enzymes and cell viability, thereby protecting the myocardium against isoproterenol-induced myocardial infarction. The decreased infarct size associated with inhibited apoptosis and increased respiratory marker enzymes provide insight on the role of ellagic acid in antiapoptotic mechanism, and it may be the reason for its cardioprotective activity.







Similar content being viewed by others
References
Sparagna, G. C., Chicco, A. J., Murphy, R. C., Bristow, M. R., Johnson, C. A., Rees, M. L., et al. (2007). Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. Journal of Lipid Research, 48, 1559–1570.
Krijnen, P. A., Nijmeijer, R., Meijer, C. J., Visser, C. A., Hack, C. E., & Niessen, H. W. (2002). Apoptosis in myocardial ischaemia and infarction. Journal of Clinical Pathology, 55, 801–811.
Hare, J. M. (2001). Oxidative stress and apoptosis in heart failure progression. Circulation Research, 89, 198–200.
Huang, J., Ito, Y., Morikawa, M., Kobune, M., Sasaki, K., Abe, T., et al. (2003). Bcl-xL gene transfer protects the heart against ischemia/reperfusion injury. Biochemical and Biophysical Research and Communications, 311, 64–70.
Vatner, D. E., Asai, K., Iwase, M., Ishikawa, Y., Shannon, R. P., Homcy, C. J., et al. (1999). Beta-adrenergic receptor-G protein-adenylyl cyclase signal transduction in the failing heart. American Journal of Cardiology, 83, 80H–85H.
Kukin, M. L., Kalman, J., Charney, R. H., Levy, D. K., Buchholz-Varley, C., Ocampo, O. N., et al. (1999). Prospective, randomized comparison of effect of long-term treatment with metoprolol or carvedilol on symptoms, exercise, ejection fraction, and oxidative stress in heart failure. Circulation, 99, 2645–2651.
Nirmala, C., & Puvanakrishnan, R. (1996). Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Molecular and Cellular Biochemistry, 159, 85–93.
Yüce, A., Ateşşahin, A., Çeribaşı, A. O., & Aksakal, M. (2007). Ellagic acid prevents cisplatin induced oxidative stress in liver and heart tissue of rats. Basic & Clinical Pharmacology & Toxicology, 101, 345–349.
Türk, G., Ateşşahin, A., Sönmez, M., Çeribaşı, A. O., & Yüce, A. (2008). Improvement of cisplatin induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertility and Sterility, 89, 1474–1481.
Aggarwal, B. B., & Shishoida, S. (2006). Molecular targets of dietary agents for prevention and therapy of cancer. Biochemical Pharmacology, 71, 1397–1421.
Mari Kannan, M., & Darlin Quine, S. (2011). Ellagic acid ameliorates isoproterenol induced oxidative stress: evidences from electrocardiological, biochemical and histological studies. European Journal of Pharmacology, 659, 45–52.
Punithavathi, V. R., & Prince, P. S. M. (2010). Pretreatment with a combination of quercetin and alpha-tocopherol ameliorates adenosine triphosphatases and lysosomal enzymes in myocardial infarcted rats. Life Sciences, 86, 178–184.
Ojha, N., Roy, S., Radtke, J., Simonetti, O., Gnyawali, S., Zweier, J. L., et al. (2008). Characterization of the structural and functional changes in the myocardium following focal ischemia–reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology, 294, H2435–H2443.
Takasawa, M., Hayakawa, M., Sugiyama, S., Hattori, K., Ito, T., & Ozawa, T. (1993). Age-associated damage in mitochondrial function in rat hearts. Experimental Gerontology, 28, 269–280.
Minakami, S., Ringler, R. L., & Singer, T. P. (1962). Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase: assay of the enzyme in particulate and in soluble preparations. The Journal of Biological Chemistry, 237, 569–576.
Pearl, W., Cascarano, J., & Zweifach, B. W. (1963). Microdetermination of cytochrome oxidase in rat tissues by oxidation on N-phenyl-p-phenylene diamine or ascorbic acid. The Journal of Histochemistry and Cytochemistry, 11, 102–104.
Kato, F., Tanaka, M., & Nakamura, K. (1999). Rapid fluorometric assay for cell viability and cell growth using nucleic acid staining and cell lysis agents. Toxicology in Vitro, 13, 923–929.
Strober, W. (1997). Trypan blue exclusion test of cell viability, in Current protocols in immunology (Coico, R., ed) (pp. A.3B.1–A.3B). Wiley, London.
Baks, T., van Geuns, R. J., Biagini, E., Wielopolski, P., Mollet, N. R., Cademartiri, F., et al. (2005). Recovery of left ventricular function after primary angioplasty for acute myocardial infarction. European Heart Journal, 26, 1070–1077.
Miura, T., & Miki, T. (2008). Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Research in Cardiology, 103, 501–513.
Altman, F. P. (1976). Tetrazolium salts and formazans. Progress in Histochemistry and Cytochemistry, 9, 1–56.
Nachlas, M. M., & Shnitka, T. K. (1963). Macroscopic identification of early myocardial infarcts by alterations in dehydrogenase activity. The American Journal of Pathology, 42, 379–405.
Ramkissoon, R. A. (1966). Macroscopic identification of early myocardial infarction by dehydrogenase alterations. Journal of Clinical Pathology, 19, 479–481.
Stanely Mainzen Prince, P., & Priya, S. (2010). Preventive effects of rutin on lysosomal enzymes in isoproterenol induced cardio toxic rats: biochemical, histological and in vitro evidences. European Journal of Pharmacology, 649, 229–235.
Pari, L., & Sivasankari, R. (2008). Effect of ellagic acid on cyclosporine A-induced oxidative damage in the liver of rats. Fundamental and Clinical Pharmacology, 22, 395–401.
Nihal, M., Ahmad, N., Mukhtar, H., & Wood, G. S. (2005). Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: Possible implications for the chemoprevention of melanoma. International Journal of Cancer, 114, 513–521.
Oltvai, Z. N., Milliman, C. L., & Korsmeyer, S. J. (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell, 74, 609–619.
Ling, H., & Lou, Y. (2005). Total flavones from Elsholtzia blanda reduce infarct size during acute myocardial ischemia by inhibiting myocardial apoptosis in rats. Journal of Ethnopharmacology, 101, 169–175.
Prabhu, S. D., Wang, G., Luo, J., Gu, Y., Ping, P., & Chandrasekar, B. (2003). Beta-adrenergic receptor blockade modulates Bcl-X(S) expression and reduces apoptosis in failing myocardium. Journal of Molecular and Cellular Cardiology, 35, 483–493.
Kawai, K., Qin, F., Shite, J., Mao, W., Fukuoka, S., & Liang, C. S. (2004). Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. American Journal of Physiology. Heart and Circulatory Physiology, 287, H1003–H1012.
Thomberry, N. A., & Lazebnik, Y. (1998). Caspases: Enemies within. Science, 281, 1312–1316.
Zou, H., Henzel, W. J., Liu, X., Lutschg, A., & Wang, X. (1997). Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell, 90, 405–413.
Zou, H., Li, Y., Liu, X., & Wang, X. (1994). An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. Journal of Biological Chemistry, 274, 11549–11556.
Hwang, J. M., Cho, J. S., Kim, T. H., & Lee, Y. I. (2010). Ellagic acid protects hepatocytes from damage by inhibiting mitochondrial production of reactive oxygen species. Biomedicine and Pharmacotherapy, 64, 264–270.
Malhotra, R., Lin, Z., Vincenz, C., & Brosius, F. C., 3rd. (2001). Hypoxia induces apoptosis via two independent pathways in Jurkat cells: differential regulation by glucose. American Journal of Physiology. Cell Physiology, 281, C1596–C1603.
Susin, S. A., Lorenzo, H. K., Zamzami, N., Marzo, I., Snow, B. E., Brothers, G. M., et al. (1999). Molecular characterization of mitochondrial apoptosis-inducing factor. Nature, 397, 441–446.
Senthilnathan, P., Padmavathi, R., Magesh, V., & Sakthisekaran, D. (2006). Modulation of TCA cycle enzymes and electron transport chain systems in experimental lung cancer. Life Sciences, 78, 1010–1014.
Vijayapadma, V., & Shyamaladevi, C. S. (2001). Effect of fish oil on mitochondrial respiration in isoproterenol induced myocardial infarction in rats. Indian Journal of Experimental Biology, 40, 268–272.
Ou, H. C., Lee, W. J., Lee, S. D., Huang, C. Y., Chiu, T. H., Tsai, K. L., et al. (2010). Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3 K/Akt/eNOS pathway. Toxicology and Applied Pharmacology, 248, 134–143.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mari Kannan, M., Darlin Quine, S. Mechanistic Clues in the Protective Effect of Ellagic Acid Against Apoptosis and Decreased Mitochondrial Respiratory Enzyme Activities in Myocardial Infarcted Rats. Cardiovasc Toxicol 12, 56–63 (2012). https://doi.org/10.1007/s12012-011-9138-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9138-7