Abstract
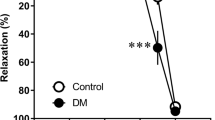
Studies have shown that tetracycline class antibiotics exhibit an ameliorating action with its antioxidant property on increased oxidative stress in tissues, including heart. Since endothelial vascular dysfunction in diabetes is associated with increased oxidative stress and prevented with antioxidants, herein, we aimed to test a hypothesis whether a low-dose doxycycline treatment of diabetic rats for 4 weeks can ameliorate endothelial vascular dysfunction of thoracic aortas. Results of the present study shows that both direct and alpha receptor-mediated contractile responses as well as endothelium-dependent and endothelium-independent vasodilatory responses were preserved with low-dose doxycycline treatment (30 μmol/kg, daily; for 4 weeks) compared with untreated diabetic group. Furthermore, doxycycline treatment normalized increased lipid peroxidation and cellular glutathione level measured in plasma and prevented diabetes-induced impaired body weight gain without significant effect on high blood glucose level. Increased membrane protein level of caveolin-1, elevated ratio of PKC in particulate and cytosolic fraction, and increased protein level of cytosolic endothelin-1 in diabetic rats were also significantly prevented with doxycycline treatment. Moreover, diabetes-induced another type of oxidative stress markers in rats, matrix metalloproteinases, MMP-2, and MMP-9 were also normalized with doxycycline treatment in blood. Taken together, our data address that amelioration and/or prevention of vascular endothelial and contractile dysfunction by doxycycline is accompanied by a clear reduction in oxidative stress markers of diabetes, which provides evidence for doxycycline’s potential antioxidant action as a therapeutic agent for amelioration and/or prevention of vascular disorders in diabetic subjects.






Similar content being viewed by others
References
Kannel, W. B., & McGee, D. L. (1979). Diabetes and cardiovascular risk factors: The framingham study. Circulation, 59, 8–13.
Johnstone, M. T., Creager, S. J., Scales, K. M., Cusco, J. A., Lee, B. K., & Creager, M. A. (1993). Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation, 88(6), 2510–2516.
Lin, T. K., Chen, S. D., Wang, P. W., Wei, Y. H., Lee, C. F., Chen, T. L., et al. (2005). Increased oxidative damage with altered antioxidative status in type 2 diabetic patients harboring the 16189 T–C variant of mitochondrial DNA. Annals of the New York Academy of Sciences, 1042, 64–69.
Khan, M. A., Thompson, C. S., Dashwood, M. R., Mumtaz, F. H., Morgan, R. J., & Mikhailidis, D. P. (2002). Endothelin-1 and nitric oxide in the pathogenesis of urinary tract disorders secondary to bladder outlet obstruction. Current Vascular Pharmacology, 1, 27–31.
Shaul, P. W., & Anderson, R. G. (1998). Role of plasmalemmal caveolae in signal transduction. American Journal of Physiology, 275(5 Pt 1), L843–L851.
Lam, T. Y., Seto, S. W., Lau, Y. M., Au, L. S., Kwan, Y. W., Ngai, S. M., et al. (2006). Impairment of the vascular relaxation and differential expression of caveolin-1 of the aorta of diabetic +db/+db mice. European Journal of Pharmacology, 546, 134–141.
Farhangkhoee, H., Khan, Z. A., Kaur, H., Xin, X., Chen, S., & Chakrabarti, S. (2006). Vascular endothelial dysfunction in diabetic cardiomyopathy: Pathogenesis and potential treatment targets. Pharmacology and Therapeutics, 11, 384–399.
Pieper, G. M., & Gross, G. J. (1988). Oxygen free radical abolish endothelium-dependent relaxation in diabetic rat aorta. American Journal of Physiology, 255, H825–H833.
Son, S. M. (2007). Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Research and Clinical Practice, 77, S65–S70.
Kamata, K., Miyata, N., Abiru, T., & Kasuya, Y. (1997). Functional changes in vascular smooth muscle and endothelium of arteries during diabetes mellitus. Life Sciences, 50(19), 1379–1387.
Kuzuya, M., Asai, T., Kanda, S., Maeda, K., Cheng, X. W., & Iguchi, A. (2001). Glycation cross-links inhibit matrix metalloproteinase-2 activation in vascular smooth muscle cells cultured on collagen lattice. Diabetologia, 44(4), 433–436.
Portik-Dobos, V., Anstadt, M. P., Hutchinson, J., Bannan, M., & Ergul, A. (2002). Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes, 51(10), 3063–3068.
Fukuda, G., Khan, Z. A., Barbin, Y. P., Farhangkhoee, H., Tilton, R. G., & Chakrabarti, S. (2005). Endothelin-mediated remodeling in aortas of diabetic rats. Diabetes Metabolism Research and Reviews, 21(4), 367–375.
Radomski, A., Sawicki, G., Olson, D. M., & Radomski, M. W. (1998). The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. British Journal of Pharmacology, 125, 1455–1462.
Zeydanli, E. N., Bilginoglu, A., Tanriverdi, E., Gurdal, H., & Turan, B. (2010). Selenium restores defective beta-adrenergic receptor response of thoracic aorta in diabetic rats. Molecular and Cellular Biochemistry, 338, 191–201.
Zeydanli, E. N., & Turan, B. (2009). Omega-3E treatment regulates matrix metalloproteinases and prevents vascular reactivity alterations in diabetic rat aorta. Canadian Journal of Physiology and Pharmacology, 87, 1063–1073.
Cohen, R. A., Tesfamariam, B., Weisbrod, R. M., & Zitnay, K. M. (1990). Adrenergic denervation in rabbits with diabetes mellitus. American Journal of Physiology, 259(1 Pt 2), H55–H61.
Pfaffman, M. A., Ball, C. R., Darby, A., & Hilman, R. (1982). Insulin reversal of diabetes-induced inhibition of vascular contractility in the rat. American Journal of Physiology, 242(4), H490–H495.
Kobayashi, T., & Kamata, K. (1999). Effect of insulin treatment on smooth muscle contractility and endothelium-dependent relaxation in rat aortae from established STZ-induced diabetes. British Journal of Pharmacology, 127(4), 835–842.
Oudot, A., Behr-Roussel, D., Compagnie, S., Caisey, S., Le Coz, O., Gorny, D., et al. (2009). Endothelial dysfunction in insulin-resistant rats is associated with oxidative stress and COX pathway dysregulation. Physiological Research, 58(4), 499–509.
Elcioglu, K. H., Kabasakal, L., Cetinel, S., Conturk, G., Sezen, S. F., & Ayanoğlu-Dülger, G. (2010). Changes in caveolin-1 expression and vasoreactivity in the aorta and corpus cavernosum of fructose and streptozotocin-induced diabetic rats. European Journal of Pharmacology, 642(1–3), 113–120.
Golub, L. M., Lee, H. M., Ryan, M. E., Giannobile, W. V., Payne, J., & Sorsa, T. (1998). Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Advances in Dental Research, 12, 12–26.
Leung, D. W., Lindlief, L. A., Laabich, A., Vissvesvaran, G. P., Kamat, M., Lieu, K. L., et al. (2007). Minocycline protects photoreceptors from light and oxidative stress in primary bovine retinal cell culture. Investigative Ophthalmology and Visual Science, 48(1), 412–421.
Kraus, R. L., Pasieczny, R., Lariosa-Willingham, K., Turner, M. S., Jiang, A., & Trauger, J. W. (2005). Antioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activity. Journal of Neurochemistry, 94(3), 819–827.
Hoyt, J. C., Ballering, J., Numanami, H., Hayden, J. M., & Robbins, R. A. (2006). Doxycycline modulates nitric oxide production in murine lung epithelial cells. Journal of Immunology, 176(1), 567–572.
Yeh, Y. C., Lai, H. C., Ting, C. T., Lee, W. L., Wang, L. C., Wang, K. Y., et al. (2007). Protection by doxycycline against doxorubicin-induced oxidative stress and apoptosis in mouse testes. Biochemical Pharmacology, 74(7), 969–980.
Lai, H. C., Yeh, Y. C., Ting, C. T., Lee, W. L., Lee, H. W., Wang, L. C., et al. (2010). Doxycycline suppresses doxorubicin-induced oxidative stress and cellular apoptosis in mouse hearts. European Journal of Pharmacology, 644(1–3), 176–187.
Yaras, N., Sariahmetoglu, M., Bilginoglu, A., Aydemir-Koksoy, A., Onay-Besikci, A., Turan, B., et al. (2008). Protective action of doxycycline against diabetic cardiomyopathy in rats. British Journal of Pharmacology, 155(8), 1174–1184.
Aydemir-Koksoy, A., Bilginoglu, A., Sariahmetoglu, M., Schulz, R., & Turan, B. (2010). Antioxidant treatment protects diabetic rats from cardiac dysfunction by preserving contractile protein targets of oxidative stress. Journal of Nutritional Biochemistry, 21(9), 827–833.
Tunctan, B., Okur, H., Calisir, C. H., Abacoglu, H., Cakici, I., Kanzik, I., et al. (1998). Comparison of nitrite oxide production by monocyte/macrophages in healthy subjects and patients with active pulmonary tuberculosis. Pharmacological Research, 37, 219–226.
Bucci, M., Roviezzo, F., Brancaleone, V., Lin, M. I., Di Lorenzo, A., Cicala, C., et al. (2004). Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(4), 721–726.
Wasan, K. M., Ng, S. P., Wong, W., & Rodrigues, B. B. (1998). Streptozotocin- and alloxan-induced diabetes modifies total plasma and lipoprotein lipid concentration and composition without altering cholesteryl ester transfer activity. Pharmacology and Toxicology, 83, 169–175.
Ak, G., Buyukberber, S., Sevinc, A., Turk, H. M., Ates, M., Sari, R., et al. (2001). The relation between plasma endothelin-1 levels and metabolic control, risk factors, treatment modalities, and diabetic microangiopathy in patients with type 2 diabetes mellitus. Journal of Diabetes Complications, 15, 150–157.
de Kreutzenberg, S. V., Crepaldi, C., Marchetto, S., Calo, L., Tiengo, A., Del Prato, S., et al. (2000). Plasma free fatty acids and endothelium-dependent vasodilation: Effect of chain-length and cyclooxygenase inhibition. Journal of Clinical Endocrinology and Metabolism, 85, 793–798.
Hattori, Y., Kawasaki, H., Abe, K., & Kanno, M. (1991). Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. American Journal of Physiology, 261(4 Pt 2), H1086–H1094.
Morrison, J., Knoll, K., Hessner, M. J., & Liang, M. (2004). Effect of high glucose on gene expression in mesangial cells: Upregulation of the thiol pathway is an adaptational response. Physiological Genomics, 17(3), 271–282.
Parthiban, A., Vijayalingam, S., Shanmugasundaram, K. R., & Mohan, R. (1995). Oxidative stress and the development of diabetic complications-antioxidants and lipid peroxidation in erythrocytes and cell membrane. Cell Biology International, 19(12), 987–993.
Rodrigues, S. F., Tran, E. D., Fortes, Z. B., & Schmid-Schönbein, G. W. (2010). Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. American Journal of Physiology Heart and Circulatory Physiology, 299(1), H25–H35.
Inoguchi, T., Li, P., Umeda, F., Yu, H. Y., Kakimoto, M., Imamura, M., et al. (2000). High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes, 49, 1939–1945.
Kamata, K., Miyata, N., Abiru, T., & Kasuya, Y. (1992). Functional changes in vascular smooth muscle and endothelium of arteries during diabetes mellitus. Life Sciences, 50, 1379–1387.
Lee, T. S., Saltsman, K. A., Ohashi, H., & King, G. L. (1989). Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proceedings of the National Academy of Science, 86, 5141–5145.
Chisolm, G. M., & Steinberg, D. (2000). The oxidative modification hypothesis of atherogenesis: an overview. Free Radical Biology and Medicine, 28, 1815–1826.
Bendeck, M. P., Conte, M., Zhang, M., Nili, N., Strauss, B. H., & Farwell, S. M. (2002). Doxycycline modulates smooth muscle cell growth, migration and matrix remodeling after arterial injury. American Journal of Pathology, 160(3), 1089–1095.
Clements, R. T., Sodha, N. R., Feng, J., Boodhwani, M., Liu, Y., Mieno, S., et al. (2009). Impaired coronary microvascular dilation correlates with enhanced vascular smooth muscle MLC phosphorylation in diabetes. Microcirculation, 16, 193–206.
Lavrentyev, E. N., & Malik, K. U. (2009). High glucose-induced Nox1-derived superoxides downregulate PKC-betaII, which subsequently decreases ACE2 expression and ANG(1–7) formation in rat VSMCs. American Journal of Physiology Heart and Circulatory Physiology, 296(1), H106–H118.
Ishii, H., Jirousek, M. R., Koya, D., Takagi, C., Xia, P., Clermont, A., et al. (1996). Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science, 272, 728–731.
Puyraimond, A., Fridman, R., Lemesle, M., Arbeille, B., & Menashi, S. (2001). MMP-2 colocalizes with caveolae on the surface of endothelial cells. Experiemntal Cell Research, 262(1), 28–36.
Chow, A. K., Cena, J., El-Yazbi, A. F., Crawford, B. D., Holt, A., Cho, W. J., et al. (2007). Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. Journal of Molecular and Cellular Cardiology, 42, 896–901.
Castro, M. M., Rizzi, E., Figueiredo-Lopes, L., Fernandes, K., Bendhack, L. M., Pitol, D. L., et al. (2008). Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis, 198, 320–331.
Akamatsu, H., Asada, M., Komura, J., Asada, Y., & Niwa, Y. (1992). Effect of doxycycline on the generation of reactive oxygen species: A possible mechanism of action of acne therapy with doxycycline. Acta Dermato-Venereologica, 72, 178–179.
Soory, M. (2008). A role for non-antimicrobial actions of tetracyclines in combating oxidative stress in periodontal and metabolic diseases: A literature review. Open Dentistry Journal, 2, 5–12.
Acknowledgments
This work has been supported by grants from TUBITAK-SBAG-107S427 to BT.
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeydanli, E.N., Kandilci, H.B. & Turan, B. Doxycycline Ameliorates Vascular Endothelial and Contractile Dysfunction in the Thoracic Aorta of Diabetic Rats. Cardiovasc Toxicol 11, 134–147 (2011). https://doi.org/10.1007/s12012-011-9107-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9107-1