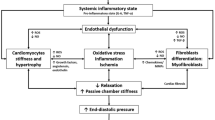
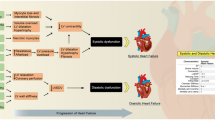
Abstract
Heart failure (HF) is characterized as a limitation to cardiac output that prevents the heart from supplying tissues with adequate oxygen and predisposes individuals to pulmonary edema. Impaired cardiac function is secondary to either decreased contractility reducing ejection (systolic failure), diminished ventricular compliance preventing filling (diastolic failure), or both. To study HF etiology, many different techniques have been developed to elicit this condition in experimental animals, with varying degrees of success. Among rats, surgically induced HF models are the most prevalent, but they bear several shortcomings, including high mortality rates and limited recapitulation of the pathophysiology, etiology, and progression of human HF. Alternatively, a number of non-invasive HF induction methods avoid many of these pitfalls, and their merits in technical simplicity, reliability, survivability, and comparability to the pathophysiologic and pathogenic characteristics of HF are reviewed herein. In particular, this review focuses on the primary pathogenic mechanisms common to genetic strains (spontaneously hypertensive and spontaneously hypertensive heart failure), pharmacological models of toxic cardiomyopathy (doxorubicin and isoproterenol), and dietary salt models, all of which have been shown to induce left ventricular HF in the rat. Additional non-invasive techniques that may potentially enable the development of new HF models are also discussed.

Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ANG II:
-
Angiotensin II
- βAR:
-
Beta adrenergic receptor
- CO:
-
Cardiac output
- DM:
-
Diabetes mellitus
- DOX:
-
Doxorubicin
- dP/dt max :
-
Peak rate of increase in LV pressure
- dP/dt min :
-
Peak rate of decrease in LV pressure
- DOCA:
-
Deoxycorticosterone acetate
- DS:
-
Dahl salt sensitive
- E/A:
-
Ratio of early-to-late inflow velocities
- EF:
-
Ejection fraction
- ESV:
-
End-systolic volume
- FS:
-
Fractional shortening
- HF:
-
Heart failure
- HR:
-
Heart rate
- i.v. :
-
Intravenous
- ISO:
-
Isoproterenol
- LAD:
-
Left anterior descending coronary artery
- LV:
-
Left ventricular
- LVP:
-
LV pressure
- LVEDP:
-
LV end-diastolic pressure
- LVESP:
-
LV end-systolic pressure
- LVOT:
-
LV outflow tract
- MAP:
-
Mean arterial pressure
- MHC:
-
Myosin heavy chain
- MI:
-
Myocardial infarction
- PO:
-
Pressure overload
- PTU:
-
Propylthiouracil
- s.c. :
-
Subcutaneous
- SD:
-
Sprauge Dawley
- SERCA:
-
Sarcoplasmic reticulum Ca2+ ATPase pump
- SERCA2a:
-
SERCA type “2”, isoform ‘a’
- SH:
-
Spontaneously hypertensive
- SHHF:
-
Spontaneously hypertensive heart failure
- SHR:
-
Spontaneously hypertensive rat
- SV:
-
Stroke volume
- T3 :
-
Triiodothyronine
- T4 :
-
Thyroxine
- TAC:
-
Transverse aortic constriction
- TNF-α:
-
Tumor necrosis factor-α
- UPS:
-
Ubiquitin–proteasome system
- VO:
-
Volume overload
References
DeFrances, C. J. & Podgornik, M. N. (2006). 2004 National hospital discharge survey. In Advance data. United States, pp. 1–19.
Minino, A. M., Heron, M. P., & Smith, B. L. (2006). Deaths: preliminary data for 2004. National vital statistics reports: From the centers for disease control and prevention, National center for health statistics. National Vital Statistics System, 54(19), 1–49.
Rosamond, W., et al. (2008). Heart disease and stroke statistics—2008 update: A report from the American heart association statistics committee and stroke statistics subcommittee. Circulation, 117(4), e25–e146.
Coronel, R., de Groot, J. R., & van Lieshout, J. J. (2001). Defining heart failure. Cardiovascular Research, 50(3), 419–422.
Lloyd-Jones, D., et al. (2009). Heart disease and stroke statistics—2009 update: A report from the American heart association statistics committee and stroke statistics subcommittee. Circulation, 119(3), e21–e181.
Goff, D. C., Jr., et al. (2000). Congestive heart failure in the United States: Is there more than meets the I(CD code)? The corpus christi heart project. In Archives of internal medicine, USA, pp. 197–202.
Balakumar, P., Singh, A. P., & Singh, M. (2007). Rodent models of heart failure. Journal of Pharmacological and Toxicological Methods, 56(1), 1–10.
Francis, G. S. (2001). Pathophysiology of chronic heart failure. American Journal of Medicine, 110(Suppl 7A), 37S–46S.
Braunwald, E., Ross, J. & Sonnenblick, E. (1976). Mechanisms of contraction of the normal and failing heart, vol. 2, p. 417. Boston: Little, Brown and Company.
Mann, D. L., & Bristow, M. R. (2005). Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation, 111(21), 2837–2849.
Diwan, A. & Dorn, G. W. II (2007). Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda), 22, 56–64.
Frey, N. & Olson, E. N. (2003). Cardiac hypertrophy: The good, the bad, and the ugly. Annual Review of Physiology, 65, 45–79.
Schoen, F. J. (2005). In V. Kumar, A. K. Abbas, & N. Fausto (Eds.), The heart, in robbins and cotran pathologic basis of disease. Elsevier Saunders: Philadelphia, PA, pp. 555–618.
Kang, Y. J. (2006). Cardiac hypertrophy: A risk factor for QT-prolongation and cardiac sudden death. Toxicologic Pathology, 34(1), 58–66.
Funada, J., et al. (2009). Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS One, 4(10), p. e7533.
Zile, M. R., et al. (2010). Mode of death in patients with heart failure and a preserved ejection fraction: Results from the irbesartan in heart failure with preserved ejection fraction study (I-Preserve) trial. Circulation, 121(12), 1393–1405.
Jackson, G., et al. (2000). ABC of heart failure. Pathophysiology. BMJ, 320(7228), 167–170.
Tonnessen, T., et al. (1997). Increased cardiac expression of endothelin-1 mRNA in ischemic heart failure in rats. Cardiovascular Research, 33(3), 601–610.
Sjaastad, I., et al. (2000). Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. Journal of Applied Physiology, 89(4), 1445–1454.
Morgan, E. E., et al. (2004). Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology, 287(5), H2049–H2053.
Holt, E., et al. (1998). Mechanisms of cardiomyocyte dysfunction in heart failure following myocardial infarction in rats. Journal of Molecular and Cellular Cardiology, 30(8), 1581–1593.
Wake, R., et al. (2005). Beneficial effect of candesartan on rat diastolic heart failure. Journal of Pharmacological Science, 98(4), 372–379.
Itter, G., et al. (2004). A model of chronic heart failure in spontaneous hypertensive rats (SHR). Laboratory Animals, 38(2), 138–148.
Pocock, S. J., et al. (2006). Predictors of mortality and morbidity in patients with chronic heart failure. European Heart Journal, 27(1), 65–75.
Janssen, B. J., et al. (2004). Effects of anesthetics on systemic hemodynamics in mice. American Journal of Physiology. Heart and Circulatory Physiology, 287(4), H1618–H1624.
Pfeffer, M. A., et al. (1979). Myocardial infarct size and ventricular function in rats. Circulation Research, 44(4), 503–512.
Opitz, C. F., et al. (1995). Arrhythmias and death after coronary artery occlusion in the rat. Continuous telemetric ECG monitoring in conscious, untethered rats. Circulation, 92(2), 253–261.
Lefebvre, F., et al. (2006). Modification of the pulmonary renin-angiotensin system and lung structural remodelling in congestive heart failure. Clinical Science (London), 111(3), pp. 217–224.
Mulder, P., et al. (1997). Role of endogenous endothelin in chronic heart failure: Effect of long-term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation, 96(6), 1976–1982.
Samsamshariat, S. A., Samsamshariat, Z. A., & Movahed, M. R. (2005). A novel method for safe and accurate left anterior descending coronary artery ligation for research in rats. Cardiovascular Revascularization Medicine, 6(3), 121–123.
Molina, E. J., et al. (2008). Novel experimental model of pressure overload hypertrophy in rats. Journal of Surgical Research.
Rockman, H. A., et al. (1991). Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proceedings of National Academic Science of USA, 88(18), 8277–8281.
Suckau, L., et al. (2009). Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation, 119(9), 1241–1252.
Weinberg, E. O., et al. (1994). Angiotensin-converting enzyme inhibition prolongs survival and modifies the transition to heart failure in rats with pressure overload hypertrophy due to ascending aortic stenosis. Circulation, 90(3), 1410–1422.
Gupta, D., et al. (2008). Adenoviral beta-adrenergic receptor kinase inhibitor gene transfer improves exercise capacity, cardiac contractility, and systemic inflammation in a model of pressure overload hypertrophy. Cardiovascular Drugs and Therapy, 22(5), 373–381.
Schwarzer, M., et al. (2009). The metabolic modulators, Etomoxir and NVP-LAB121, fail to reverse pressure overload induced heart failure in vivo. Basic Research in Cardiology, 104(5), 547–557.
Cantor, E. J., et al. (2005). A comparative serial echocardiographic analysis of cardiac structure and function in rats subjected to pressure or volume overload. Journal of Molecular and Cellular Cardiology, 38(5), 777–786.
Rivera, D. M., & Lowes, B. D. (2005). Molecular remodeling in the failing human heart. Current Heart Failure Reports, 2(1), 5–9.
del Monte, F., et al. (2001). Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2 +)-ATPase in a rat model of heart failure. Circulation, 104(12), 1424–1429.
Gianni, D., et al. (2005). SERCA2a in heart failure: Role and therapeutic prospects. Journal of Bioenergetics and Biomembranes, 37(6), 375–380.
Tsukioka, T., et al. (2007). Local and systemic impacts of pleural oxygen exposure in thoracotomy. BioFactors, 30(2), 117–128.
Buvanendran, A., et al. (2004). Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesthesia and Analgesia, 99(5), 1453–1460; table of contents.
del Monte, F., et al. (2002). Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics, 9(1), 49–56.
Lebeche, D., et al. (2004). In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation, 110(22), 3435–3443.
Hu, P., et al. (2003). Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. American Journal of Physiology. Heart and Circulatory Physiology, 285(3), H1261–H1269.
Stansfield, W. E., et al. (2007). Characterization of a model to independently study regression of ventricular hypertrophy. Journal of Surgical Research, 142(2), 387–393.
Pawlush, D. G., et al. (1993). Echocardiographic evaluation of size, function, and mass of normal and hypertrophied rat ventricles. Journal of Applied Physiology, 74(5), 2598–2605.
Kobayashi, S., et al. (1996). Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation, 94(12), 3362–3368.
Shah, K. B., et al. (2009). The cardioprotective effects of fish oil during pressure overload are blocked by high fat intake: Role of cardiac phospholipid remodeling. Hypertension, 54(3), 605–611.
Linz, W., et al. (1996). ACE inhibition decreases postoperative mortality in rats with left ventricular hypertrophy and myocardial infarction. Clinical and Experimental Hypertension, 18(5), 691–712.
Luo, J. D., et al. (1999). Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clinical and Experimental Pharmacology and Physiology, 26(11), 903–908.
Woodiwiss, A. J., et al. (2001). Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation, 103(1), 155–160.
Sethi, R., et al. (2007). Dependence of changes in beta-adrenoceptor signal transduction on type and stage of cardiac hypertrophy. Journal of Applied Physiology, 102(3), 978–984.
Monnet, E., & Orton, E. C. (1999). A canine model of heart failure by intracoronary adriamycin injection: Hemodynamic and energetic results. Journal of Cardiac Failure, 5(3), 255–264.
Wang, X., et al. (2003). Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. Journal of Applied Physiology, 94(2), 752–763.
Brower, G. L., & Janicki, J. S. (2001). Contribution of ventricular remodeling to pathogenesis of heart failure in rats. American Journal of Physiology. Heart and Circulatory Physiology, 280(2), H674–H683.
Janicki, J. S., et al. (2006). Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovascular Research, 69(3), 657–665.
Gardner, J. D., Brower, G. L., & Janicki, J. S. (2002). Gender differences in cardiac remodeling secondary to chronic volume overload. Journal of Cardiac Failure, 8(2), 101–107.
Brower, G. L., Henegar, J. R. & Janicki, J. S. (1996). Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. American Journal of Physiology. Heart and Circulatory Physiology, 271(5 Pt 2), pp. H2071–H2078.
Moreno, J., et al. (2005). Effect of remodelling, stretch and ischaemia on ventricular fibrillation frequency and dynamics in a heart failure model. Cardiovascular Research, 65(1), 158–166.
Hanna, N., et al. (2004). Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovascular Research, 63(2), 236–244.
Cardin, S., et al. (2003). Evolution of the atrial fibrillation substrate in experimental congestive heart failure: Angiotensin-dependent and -independent pathways. Cardiovascular Research, 60(2), 315–325.
Takagaki, M., et al. (2002). Induction and maintenance of an experimental model of severe cardiomyopathy with a novel protocol of rapid ventricular pacing. Journal of Thoracic and Cardiovascular Surgery, 123(3), 544–549.
Monnet, E., & Chachques, J. C. (2005). Animal models of heart failure: What is new? Annals of Thoracic Surgery, 79(4), 1445–1453.
Chekanov, V. S., et al. (2000). Effects of electrical stimulation postcardiomyoplasty in a model of chronic heart failure: Hemodynamic results after daily 12-hour cessation versus a nonstop regimen. Pacing and Clinical Electrophysiology, 23(7), 1094–1102.
Hasenfuss, G. (1998). Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovascular Research, 39(1), 60–76.
Maass, A. H., et al. (2004). Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation, 110(15), 2102–2109.
van Rooij, E., et al. (2007). Control of stress-dependent cardiac growth and gene expression by a microRNA. Science, 316(5824), 575–579.
de Resende, M. M., Kriegel, A. J., & Greene, A. S. (2006). Combined effects of low-dose spironolactone and captopril therapy in a rat model of genetic hypertrophic cardiomyopathy. Journal of Cardiovascular Pharmacology, 48(6), 265–273.
Muller, D. N., Derer, W., & Dechend, R. (2008). Aliskiren—Mode of action and preclinical data. Journal of Molecular Medicine, 86(6), 659–662.
Wichers, L. B., et al. (2004). Effects of instilled combustion-derived particles in spontaneously hypertensive rats. Part I: Cardiovascular responses. Inhalation Toxicology, 16(6–7), 391–405.
Calhoun, D. A., et al. (1994). Diurnal blood pressure variation and dietary salt in spontaneously hypertensive rats. Hypertension, 24(1), 1–7.
El-Mas, M. M., & Abdel-Rahman, A. A. (2005). Longitudinal studies on the effect of hypertension on circadian hemodynamic and autonomic rhythms in telemetered rats. Life Science, 76(8), 901–915.
Bing, O. H., et al. (1995). The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. Journal of Molecular and Cellular Cardiology, 27(1), 383–396.
Boluyt, M. O., Bing, O. H. & Lakatta, E. G. (1995). The ageing spontaneously hypertensive rat as a model of the transition from stable compensated hypertrophy to heart failure. European Heart Journal, 16(Suppl N), pp. 19–30.
Badenhorst, D., et al. (2003). Beta-adrenergic activation initiates chamber dilatation in concentric hypertrophy. Hypertension, 41(3), 499–504.
Kuoppala, A., et al. (2003). Expression of bradykinin receptors in the left ventricles of rats with pressure overload hypertrophy and heart failure. Journal of Hypertension, 21(9), 1729–1736.
Koletsky, S. (1975). Pathologic findings and laboratory data in a new strain of obese hypertensive rats. American Journal of Pathology, 80(1), 129–142.
McCune, S., Baker, P. & Stills, H. Jr. (1990). SHHF/Mcc-cp rat: Model of obesity, non-insulin-dependent diabetes, and congestive heart failure. ILAR (Institute for Laboratory Animal Research) Journal, 32.
Muders, F., & Elsner, D. (2000). Animal models of chronic heart failure. Pharmacological Research, 41(6), 605–612.
Roncalli, J., et al. (2007). NMR and cDNA array analysis prior to heart failure reveals an increase of unsaturated lipids, a glutamine/glutamate ratio decrease and a specific transcriptome adaptation in obese rat heart. Journal of Molecular and Cellular Cardiology, 42(3), 526–539.
Mark, A. L., et al. (2003). A leptin-sympathetic-leptin feedback loop: Potential implications for regulation of arterial pressure and body fat. Acta Physiologica Scandinavica, 177(3), 345–349.
Radin, M. J., et al. (2003). Increased salt sensitivity secondary to leptin resistance in SHHF rats is mediated by endothelin. Molecular and Cellular Biochemistry, 242(1–2), 57–63.
Jackson, E. K., et al. (2001). A(1) receptor blockade induces natriuresis with a favorable renal hemodynamic profile in SHHF/Mcc-fa(cp) rats chronically treated with salt and furosemide. Journal of Pharmacology and Experimental Therapeutics, 299(3), 978–987.
McCune, S. A., et al. (1995). SHHF/Mcc-facp rat model: effects of gender and genotype on age of expression of metabolic complications and congestive heart failure and on response to drug therapy. In E. Shafir (Ed.), Lessons from animal diabetes V. Smith-Gordon: London, pp. 255–270.
Emter, C. A., et al. (2005). Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. American Journal of Physiology. Heart and Circulatory Physiology, 289(5), H2030–H2038.
Abe, Y., et al. (2007). Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. American Journal of Physiology. Heart and Circulatory Physiology, 292(5), H2387–H2396.
Bienertova-Vasku, J. A., et al. (2009). Association between variants in the genes for leptin, leptin receptor, and proopiomelanocortin with chronic heart failure in the Czech population. Heart and Vessels, 24(2), 131–137.
Haas, G. J., et al. (1995). Echocardiographic characterization of left ventricular adaptation in a genetically determined heart failure rat model. American Heart Journal, 130(4), 806–811.
Carll, A. P., et al. (2010). Particulate matter inhalation exacerbates cardiopulmonary injury in a rat model of isoproterenol-induced cardiomyopathy. Inhalation Toxicology, 22(5), 355–368.
Carll, A. P. (2010). Unpublished data.
Schlenker, E. H., Kost, C. K., Jr., & Likness, M. M. (2004). Effects of long-term captopril and l-arginine treatment on ventilation and blood pressure in obese male SHHF rats. Journal of Applied Physiology, 97(3), 1032–1039.
Poornima, I., et al. (2008). Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circulation. Heart failure, 1(3), 153–160.
Peterson, J. T., et al. (2001). Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation, 103(18), 2303–2309.
Hohl, C. M., et al. (1993). Effects of obesity and hypertension on ventricular myocytes: Comparison of cells from adult SHHF/Mcc-cp and JCR:LA-cp rats. Cardiovascular Research, 27(2), 238–242.
Heyen, J. R., et al. (2002). Structural, functional, and molecular characterization of the SHHF model of heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 283(5), H1775–H1784.
Anderson, K. M., et al. (1999). The myocardial beta-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension, 33(1 Pt 2), pp. 402–407.
Tamura, T., Said, S., & Gerdes, A. M. (1999). Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension, 33(2), 676–680.
Reffelmann, T., & Kloner, R. A. (2003). Transthoracic echocardiography in rats. Evaluation of commonly used indices of left ventricular dimensions, contractile performance, and hypertrophy in a genetic model of hypertrophic heart failure (SHHF-Mcc-facp-Rats) in comparison with Wistar rats during aging. Basic Research in Cardiology, 98(5), 275–284.
Janssen, P. M., et al. (2003). Selective contractile dysfunction of left, not right, ventricular myocardium in the SHHF rat. American Journal of Physiology. Heart and Circulatory Physiology, 284(3), H772–H778.
Onodera, T., et al. (1998). Maladaptive remodeling of cardiac myocyte shape begins long before failure in hypertension. Hypertension, 32(4), 753–757.
Gerdes, A. M., et al. (1996). Myocyte remodeling during the progression to failure in rats with hypertension. Hypertension, 28(4), 609–614.
Park, S., et al. (1997). Verapamil accelerates the transition to heart failure in obese, hypertensive, female SHHF/Mcc-fa(cp) rats. Journal of Cardiovascular Pharmacology, 29(6), 726–733.
Ferrara, C. M., et al. (1996). Exercise training and the glucose transport system in obese SHHF/Mcc-fa(cp) rats. Journal of Applied Physiology, 81(4), 1670–1676.
Pacher, P., et al. (2004). Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 287(5), H2132–H2137.
Anversa, P., et al. (1994). Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. American Journal of Physiology. Heart and Circulatory Physiology, 267(3 Pt 2), pp. H1062–H1073.
Bugger, H., & Abel, E. D. (2009). Rodent models of diabetic cardiomyopathy. Disease Models & Mechanisms, 2(9–10), 454–466.
Bristow, M. R., et al. (1980). Acute and chronic cardiovascular effects of doxorubicin in the dog: The cardiovascular pharmacology of drug-induced histamine release. Journal of Cardiovascular Pharmacology, 2(5), 487–515.
Djelmami-Hani, M., et al. (2007). Induction of heart failure: Haemodynamic comparison of three different canine models. Laboratory Animals, 41(1), 63–70.
Ferreira, A. L., Matsubara, L. S., & Matsubara, B. B. (2008). Anthracycline-induced cardiotoxicity. Cardiovascular & Hematological Agents In Medicinal Chemistry, 6(4), 278–281.
Gille, L., & Nohl, H. (1997). Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radical Biology and Medicine, 23(5), 775–782.
Herman, E. H., et al. (1998). Comparison of the chronic toxicity of piroxantrone, losoxantrone and doxorubicin in spontaneously hypertensive rats. Toxicology, 128(1), 35–52.
Liu, X., et al. (2006). Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. Journal of the American College of Cardiology, 48(7), 1438–1447.
Saad, S. Y., Najjar, T. A., & Al-Rikabi, A. C. (2001). The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacological Research, 43(3), 211–218.
Arola, O. J., et al. (2000). Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Research, 60(7), 1789–1792.
Li, T., Danelisen, I., & Singal, P. K. (2002). Early changes in myocardial antioxidant enzymes in rats treated with adriamycin. Molecular and Cellular Biochemistry, 232(1–2), 19–26.
Liu, J., et al. (2008). A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome. American Journal of Physiology. Heart and Circulatory Physiology, 295(6), H2541–H2550.
Kumarapeli, A. R., et al. (2005). A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB Journal, 19(14), 2051–2053.
Tu, V. C., Bahl, J. J., & Chen, Q. M. (2002). Signals of oxidant-induced cardiomyocyte hypertrophy: Key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. Journal of Pharmacology and Experimental Therapeutics, 300(3), 1101–1110.
Olson, R. D., et al. (2005). Doxorubicin cardiac dysfunction: Effects on calcium regulatory proteins, sarcoplasmic reticulum, and triiodothyronine. Cardiovascular Toxicology, 5(3), 269–283.
Pfizer Inc. (2010). Doxorubicin Hydrochloride for Injection, USP. Available from: http://www.pfizer.com/files/products/uspi_adriamycin.pdf.
U.S. Food and Drug Administration. (2010). Oncology Tools: Dose Calculator. Available from: http://www.accessdata.fda.gov/scripts/cder/onctools/animalresults.cfm.
Hayward, R., & Hydock, D. S. (2007). Doxorubicin cardiotoxicity in the rat: An in vivo characterization. Journal of American Association of Laboratory in Animal Science, 46(4), 20–32.
Kozluca, O., et al. (1996). Prevention of doxorubicin induced cardiotoxicity by catechin. Cancer Letters, 99(1), 1–6.
Chen, X., et al. (2007). Preventive cardioprotection of erythropoietin against doxorubicin-induced cardiomyopathy. Cardiovascular Drugs and Therapy, 21(5), 367–374.
Matsui, H., et al. (1999). Protective effects of carvedilol against doxorubicin-induced cardiomyopathy in rats. Life Science, 65(12), 1265–1274.
Ueno, M., et al. (2006). Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. Journal of Pharmacological Science, 101(2), 151–158.
Deepa, P. R., & Varalakshmi, P. (2003). Protective effect of low molecular weight heparin on oxidative injury and cellular abnormalities in adriamycin-induced cardiac and hepatic toxicity. Chemico-biological Interactions, 146(2), 201–210.
Zheng, M., Han, Q. D., & Xiao, R. P. (2004). Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance. Sheng li xue bao, 56(1), 1–15.
Rockman, H. A., Koch, W. J., & Lefkowitz, R. J. (2002). Seven-transmembrane-spanning receptors and heart function. Nature, 415(6868), 206–212.
Wu, Y., et al. (2009). Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proceedings of National Academic Science USA, 106(14), 5972–5977.
Boluyt, M.O., et al., Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. American Journal of Physiology. Heart and Circulatory Physiology, 1995. 269(2 Pt 2): p. H638-47.
Zevitz, M. E. & October (2006). Heart Failure. eMedicine from WebMD.
Rona, G. (1985). Catecholamine cardiotoxicity. Journal of Molecular and Cellular Cardiology, 17(4), 291–306.
Peng, Y., et al. (2003). Effects of catecholamine-beta-adrenoceptor-cAMP system on severe patients with heart failure. Chinese Medical Journal, 116(10), 1459–1463.
Abraham, J., et al. (2009). Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. Journal of the American College of Cardiology, 53(15), 1320–1325.
Wittstein, I. S. (2008). Acute stress cardiomyopathy. Current Heart Failure Reports, 5(2), 61–68.
Iaccarino, G., et al. (2005). Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. European Heart Journal, 26(17), 1752–1758.
Iaccarino, G., et al. (1999). Bbeta-adrenergic receptor kinase-1 levels in catecholamine-induced myocardial hypertrophy: Regulation by beta- but not alpha1-adrenergic stimulation. Hypertension, 33(1 Pt 2), 396–401.
Lamba, S., & Abraham, W. T. (2000). Alterations in adrenergic receptor signaling in heart failure. Heart Failure Reviews, 5(1), 7–16.
Keys, J. R. & Koch, W. J. (2004). The adrenergic pathway and heart failure. Recent progress in hormone research, 59, 13–30.
Nishikawa, M., et al. (1993). Differential down-regulation of pulmonary beta 1- and beta 2-adrenoceptor messenger RNA with prolonged in vivo infusion of isoprenaline. European Journal of Pharmacology, 247(2), 131–138.
Yeager, J. C., & Iams, S. G. (1981). The hemodynamics of isoproterenol-induced cardiac failure in the rat. Circulatory Shock, 8(2), 151–163.
Maisel, A. S., et al. (1989). Regulation of cardiac beta-adrenergic receptors by captopril. Implications for congestive heart failure. Circulation, 80(3), 669–675.
Murray, D. R., Prabhu, S. D., & Chandrasekar, B. (2000). Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation, 101(20), 2338–2341.
Nerme, V., Abrahamsson, T., & Vauquelin, G. (1990). Chronic isoproterenol administration causes altered beta adrenoceptor-Gs-coupling in guinea pig lung. Journal of Pharmacology and Experimental Therapeutics, 252(3), 1341–1346.
Jia, Y. X., et al. (2006). Apelin protects myocardial injury induced by isoproterenol in rats. Regulatory Peptides, 133(1–3), 147–154.
Montgomery, R. L., et al. (2008). Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. Journal of Clinical Investigation, 118(11), 3588–3597.
Reiken, S., et al. (2003). Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. Journal of Biological Chemistry, 278(1), 444–453.
Zhang, Z. S., et al. (2005). Enhanced inhibition of L-type Ca2 + current by beta3-adrenergic stimulation in failing rat heart. Journal of Pharmacology and Experimental Therapeutics, 315(3), 1203–1211.
Borlak, J., & Thum, T. (2003). Hallmarks of ion channel gene expression in end-stage heart failure. FASEB Journal, 17(12), 1592–1608.
Grimm, D., et al. (1998). Development of heart failure following isoproterenol administration in the rat: Role of the renin-angiotensin system. Cardiovascular Research, 37(1), 91–100.
Yan, Y. H., et al. (2008). Effects of diesel exhaust particles on left ventricular function in isoproterenol-induced myocardial injury and healthy rats. Inhalation Toxicology, 20(2), 199–203.
Grimm, D., et al. (1999). Effects of beta-receptor blockade and angiotensin II type I receptor antagonism in isoproterenol-induced heart failure in the rat. Cardiovascular Pathology, 8(6), 315–323.
Brouri, F., et al. (2002). Toxic cardiac effects of catecholamines: Role of beta-adrenoceptor downregulation. European Journal of Pharmacology, 456(1–3), 69–75.
Brouri, F., et al. (2004). Blockade of beta 1- and desensitization of beta 2-adrenoceptors reduce isoprenaline-induced cardiac fibrosis. European Journal of Pharmacology, 485(1–3), 227–234.
Zhang, G. X., et al. (2005). Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovascular Research, 65(1), 230–238.
Osadchii, O. E., et al. (2007). Cardiac dilatation and pump dysfunction without intrinsic myocardial systolic failure following chronic beta-adrenoreceptor activation. American Journal of Physiology. Heart and Circulatory Physiology, 292(4), H1898–H1905.
Harden, T. K., Su, Y. F., & Perkins, J. P. (1979). Catecholamine-induced desensitization involves an uncoupling of beta-adrenergic receptors and adenylate cyclase. Journal of Cyclic Nucleotide Research, 5(2), 99–106.
Benjamin, I. J., et al. (1989). Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circulation Research, 65(3), 657–670.
Friddle, C. J., et al. (2000). Expression profiling reveals distinct sets of genes altered during induction and regression of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America, 97(12), 6745–6750.
Leenen, F. H., White, R., & Yuan, B. (2001). Isoproterenol-induced cardiac hypertrophy: Role of circulatory versus cardiac renin-angiotensin system. American Journal of Physiology. Heart and Circulatory Physiology, 281(6), H2410–H2416.
Gengo, P., et al. (1988). Regulation by chronic drug administration of neuronal and cardiac calcium channel, beta-adrenoceptor and muscarinic receptor levels. Biochemical Pharmacology, 37(4), 627–633.
Hayes, J. S., Pollock, G. D., & Fuller, R. W. (1984). In vivo cardiovascular responses to isoproterenol, dopamine and tyramine after prolonged infusion of isoproterenol. Journal of Pharmacology and Experimental Therapeutics, 231(3), 633–639.
Bos, R., et al. (2005). Inhibition of catecholamine-induced cardiac fibrosis by an aldosterone antagonist. Journal of Cardiovascular Pharmacology, 45(1), 8–13.
Oliveira, E. M., & Krieger, J. E. (2005). Chronic beta-adrenoceptor stimulation and cardiac hypertrophy with no induction of circulating renin. European Journal of Pharmacology, 520(1–3), 135–141.
Takeshita, D., et al. (2008). Isoproterenol-induced hypertrophied rat hearts: Does short-term treatment correspond to long-term treatment? The Journal of Physiological Sciences, 58(3), 179–188.
Johar, S., et al. (2006). Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB Journal, 20(9), 1546–1548.
Sakata, Y., et al. (2003). Angiotensin II type 1 receptor blockade prevents diastolic heart failure through modulation of Ca(2 +) regulatory proteins and extracellular matrix. Journal of Hypertension, 21(9), 1737–1745.
Di Zhang, A., et al. (2008). Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension, 52(6), 1060–1067.
Freund, C., et al. (2005). Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation, 111(18), 2319–2325.
Beck, A., et al. (1985). Angiotensin-induced hypertension in conscious dogs: Biochemical parameters and baroreceptor reflex. Cardiovascular Research, 19(11), 721–726.
Cao, R. Y., et al. (2010). The murine angiotensin II-induced abdominal aortic aneurysm model: Rupture risk and inflammatory progression patterns. Frontiers in Pharmacology, 1(9), 1–7.
Perrino, C., et al. (2006). Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. Journal of Clinical Investigation, 116(6), 1547–1560.
Hershman, J. M. & March (2008). Hypothyroidism: Thyroid Disorders: Merck Manual Professional. Merck.
Maitra, A. & Abbas, A. K. (2005). The endocrine system. In V. Kumar, A. K. Abbas, & N. Fausto (Eds.), Robbins and Cotran pathologic basis of disease. Philadelphia, PA: Elsevier Saunders, pp. 1155–1226.
Gay, R. G., et al. (1988). Effects of thyroid state on venous compliance and left ventricular performance in rats. American Journal of Physiology, 254(1 Pt 2), pp. H81–H88.
Tang, Y. D., et al. (2005). Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation, 112(20), 3122–3130.
Liu, Z., & Gerdes, A. M. (1990). Influence of hypothyroidism and the reversal of hypothyroidism on hemodynamics and cell size in the adult rat heart. Journal of Molecular and Cellular Cardiology, 22(12), 1339–1348.
Kisso, B., et al. (2008). Effect of low thyroid function on cardiac structure and function in spontaneously hypertensive heart failure rats. Journal of Cardiac Failure, 14(2), 167–171.
Schuyler, G. T., & Yarbrough, L. R. (1990). Changes in myosin and creatine kinase mRNA levels with cardiac hypertrophy and hypothyroidism. Basic Research in Cardiology, 85(5), 481–494.
Seta, Y., et al. (1996). Basic mechanisms in heart failure: The cytokine hypothesis. Journal of Cardiac Failure, 2(3), 243–249.
Bryant, D., et al. (1998). Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation, 97(14), 1375–1381.
Panagopoulou, P., et al. (2008). Desmin mediates TNF-alpha-induced aggregate formation and intercalated disk reorganization in heart failure. Journal of Cell Biology, 181(5), 761–775.
Prabhu, S. D. (2004). Cytokine-induced modulation of cardiac function. Circulation Research, 95(12), 1140–1153.
Mariappan, N., et al. (2007). TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. American Journal of Physiology. Heart and Circulatory Physiology, 293(5), H2726–H2737.
Bozkurt, B., et al. (1998). Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation, 97(14), 1382–1391.
Biaggioni, I. (2007). The sympathetic nervous system and blood volume regulation: Lessons from autonomic failure patients. American Journal of the Medical Sciences, 334(1), 61–64.
Gradin, K., Elam, M. & Persson, B. (1985). Chronic salt loading and central adrenergic mechanisms in the spontaneously hypertensive rat. Acta Pharmacologica et Toxicologica (Copenh), 56(3), 204–213.
Gradin, K., et al. (1988). Adrenergic mechanisms during hypertension induced by sucrose and/or salt in the spontaneously hypertensive rat. Naunyn-Schmiedebergs Arch Pharmacol, 337(1), 47–52.
Takata, Y., et al. (1988). Central and peripheral mechanisms of the enhanced hypertension following long-term salt loading in spontaneously hypertensive rats. Japanese Circulation Journal, 52(11), 1317–1322.
Ahn, J., et al. (2004). Cardiac structural and functional responses to salt loading in SHR. American Journal of Physiology. Heart and Circulatory Physiology, 287(2), H767–H772.
Varagic, J., et al. (2006). Myocardial fibrosis, impaired coronary hemodynamics, and biventricular dysfunction in salt-loaded SHR. American Journal of Physiology. Heart and Circulatory Physiology, 290(4), H1503–H1509.
Watson, P. A., et al. (2007). Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. American Journal of Physiology. Heart and Circulatory Physiology, 293(1), H246–H259.
Miyachi, M., et al. (2009). Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension, 53(4), 701–707.
Yu, H. C., et al. (1998). Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation, 98(23), 2621–2628.
Radin, M. J., et al. (2008). Salt-induced cardiac hypertrophy is independent of blood pressure and endothelin in obese, heart failure-prone SHHF rats. Clinical and Experimental Hypertension, 30(7), 541–552.
Iwanaga, Y., et al. (2001). Differential effects of angiotensin II versus endothelin-1 inhibitions in hypertrophic left ventricular myocardium during transition to heart failure. Circulation, 104(5), 606–612.
Takenaka, H., et al. (2006). Angiotensin II, oxidative stress, and extracellular matrix degradation during transition to LV failure in rats with hypertension. Journal of Molecular and Cellular Cardiology, 41(6), 989–997.
Ogata, T., et al. (2004). Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. Journal of the American College of Cardiology, 43(8), 1481–1488.
Kramer, F., et al. (2008). Plasma concentrations of matrix metalloproteinase-2, tissue inhibitor of metalloproteinase-1 and osteopontin reflect severity of heart failure in DOCA-salt hypertensive rat. Biomarkers, 13(3), 270–281.
Bjelogrlic, S. K., et al. (2007). Effects of dexrazoxane and amifostine on evolution of Doxorubicin cardiomyopathy in vivo. Experimental Biology and Medicine, 232(11), 1414–1424.
Xu, M., et al. (2008). Protective effect of the endothelin antagonist CPU0213 against isoprenaline-induced heart failure by suppressing abnormal expression of leptin, calcineurin and SERCA2a in rats. Journal of Pharmacy and Pharmacology, 60(6), 739–745.
Suzuki, M., et al. (1998). Altered inotropic response of endothelin-1 in cardiomyocytes from rats with isoproterenol-induced cardiomyopathy. Cardiovascular Research, 39(3), 589–599.
Meszaros, J., & Levai, G. (1990). Ultrastructural and electrophysiological alterations during the development of catecholamine-induced cardiac hypertrophy and failure. Acta Biologica Hungarica, 41(4), 289–307.
Teerlink, J. R., Pfeffer, J. M., & Pfeffer, M. A. (1994). Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circulation Research, 75(1), 105–113.
Bruch, C., et al. (2000). Tei-index in patients with mild-to-moderate congestive heart failure. European Heart Journal, 21(22), 1888–1895.
Kim-Mitsuyama, S., et al. (2004). Additive beneficial effects of the combination of a calcium channel blocker and an angiotensin blocker on a hypertensive rat-heart failure model. Hypertension Research, 27(10), 771–779.
Acknowledgments
The authors thank and acknowledge Drs. Urmila Kodavanti of the U.S. EPA and David Kurtz of Experimental Pathology Laboratories for their reviews of this manuscript.
Disclaimer
This paper has been reviewed and approved for release by the National Health and Environmental Effects Research Laboratory, U.S. EPA. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Declaration of interest
Alex Carll is supported by UNC/EPA CR83323601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carll, A.P., Willis, M.S., Lust, R.M. et al. Merits of Non-Invasive Rat Models of Left Ventricular Heart Failure. Cardiovasc Toxicol 11, 91–112 (2011). https://doi.org/10.1007/s12012-011-9103-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9103-5