Abstract
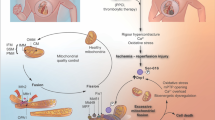
Myocardial ischemia is the main cause of death in the Western societies. Therapeutic strategies aimed to protect the ischemic myocardium have been extensively studied. Reperfusion is the definitive treatment for acute coronary syndromes, especially acute myocardial infarction; however, reperfusion has the potential to exacerbate tissue injury, a process termed reperfusion injury. Ischemia/reperfusion (I/R) injury may lead to cardiac arrhythmias and contractile dysfunction that involve apoptosis and necrosis in the heart. The present review describes the mitochondrial role on cardiomyocyte death and some potential pharmacological strategies aimed at preventing the opening of the box, i.e., mitochondrial dysfunction and membrane permeabilization that result into cell death. Data in the literature suggest that mitochondrial disruption during I/R can be avoided, although uncertainties still exist, including the fact that the optimal windows of treatment are still fairly unknown. Despite this, the protection of cardiac mitochondrial function should be critical for the patient survival, and new strategies to avoid mitochondrial alterations should be designed to avoid cardiomyocyte loss.




Similar content being viewed by others
References
Hearse, D. J. (1990). Ischemia, reperfusion, and the determinants of tissue injury. Cardiovascular Drugs and Therapy, 4(Suppl 4), 767–776.
Holleyman, C. R., & Larson, D. F. (2001). Apoptosis in the ischemic reperfused myocardium. Perfusion, 16, 491–502.
Marczin, N., El-Habashi, N., Hoare, G. S., Bundy, R. E., & Yacoub, M. (2003). Antioxidants in myocardial ischemia–reperfusion injury: Therapeutic potential and basic mechanisms. Archives of Biochemistry and Biophysics, 420, 222–236.
Ferrari, R., Curello, S., Boffa, G. M., Condorelli, E., Pasini, E., Guarnieri, G., et al. (1989). Oxygen free radical-mediated heart injury in animal models and during bypass surgery in humans. Effects of alpha-tocopherol. Annals of the New York Academy of Sciences, 570, 237–253.
Ambrosio, G., Zweier, J. L., Duilio, C., Kuppusamy, P., Santoro, G., Elia, P. P., et al. (1993). Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. The Journal of Biological Chemistry, 268, 18532–18541.
Nagy, N., Malik, G., Tosaki, A., Ho, Y. S., Maulik, N., & Das, D. K. (2008). Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. Journal of Molecular and Cellular Cardiology, 44, 252–260.
Chen, Z., Siu, B., Ho, Y. S., Vincent, R., Chua, C. C., Hamdy, R. C., et al. (1998). Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. Journal of Molecular and Cellular Cardiology, 30, 2281–2289.
Li, G., Chen, Y., Saari, J. T., & Kang, Y. J. (1997). Catalase-overexpressing transgenic mouse heart is resistant to ischemia–reperfusion injury. American Journal of Physiology, 273, H1090–H1095.
Yoshida, T., Watanabe, M., Engelman, D. T., Engelman, R. M., Schley, J. A., Maulik, N., et al. (1996). Transgenic mice overexpressing glutathione peroxidase are resistant to myocardial ischemia reperfusion injury. Journal of Molecular and Cellular Cardiology, 28, 1759–1767.
Lemieux, H., & Hoppel, C. L. (2009). Mitochondria in the human heart. Journal of Bioenergetics and Biomembranes, 41, 99–106.
Riva, A., Tandler, B., Loffredo, F., Vazquez, E., & Hoppel, C. (2005). Structural differences in two biochemically defined populations of cardiac mitochondria. American Journal of Physiology Heart and circulatory Physiology, 289, H868–H872.
Palmer, J. W., Tandler, B., & Hoppel, C. L. (1977). Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. The Journal of Biological Chemistry, 252, 8731–8739.
Palmer, J. W., Tandler, B., & Hoppel, C. L. (1985). Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: Effects of procedural manipulations. Archives of Biochemistry and Biophysics, 236, 691–702.
Lesnefsky, E. J., Slabe, T. J., Stoll, M. S., Minkler, P. E., & Hoppel, C. L. (2001). Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. American Journal of Physiology Heart and Circulatory Physiology, 280, H2770–H2778.
Lesnefsky, E. J., Tandler, B., Ye, J., Slabe, T. J., Turkaly, J., & Hoppel, C. L. (1997). Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. American Journal of Physiology, 273, H1544–H1554.
Duan, J., & Karmazyn, M. (1989). Relationship between oxidative phosphorylation and adenine nucleotide translocase activity of two populations of cardiac mitochondria and mechanical recovery of ischemic hearts following reperfusion. Canadian Journal of Physiology and Pharmacology, 67, 704–709.
Loos, B., & Engelbrecht, A. M. (2009). Cell death: A dynamic response concept. Autophagy, 5, 590–603.
Mani, K. (2008). Programmed cell death in cardiac myocytes: Strategies to maximize post-ischemic salvage. Heart Failure Reviews, 13, 193–209.
Dhesi, P., Tehrani, F., Fuess, J., & Schwarz, E. R. (2009). How does the heart (not) die? The role of autophagy in cardiomyocyte homeostasis and cell death. Heart Failure Reviews, in press.
Gustafsson, A. B., & Gottlieb, R. A. (2008). Eat your heart out: Role of autophagy in myocardial ischemia/reperfusion. Autophagy, 4, 416–421.
Hamacher-Brady, A., Brady, N. R., & Gottlieb, R. A. (2006). The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovascular Drugs and Therapy, 20, 445–462.
Lee, Y., & Gustafsson, A. B. (2009). Role of apoptosis in cardiovascular disease. Apoptosis, 14, 536–548.
Gupta, S. (2003). Molecular signaling in death receptor and mitochondrial pathways of apoptosis (Review). International Journal of Oncology, 22, 15–20.
Baines, C. P. (2009). The mitochondrial permeability transition pore and ischemia–reperfusion injury. Basic Research in Cardiology, 104, 181–188.
Riedl, S. J., & Salvesen, G. S. (2007). The apoptosome: Signalling platform of cell death. Nature Reviews Molecular Cell Biology, 8, 405–413.
Buja, L. M. (2005). Myocardial ischemia and reperfusion injury. Cardiovascular Pathology, 14, 170–175.
Freude, B., Masters, T. N., Robicsek, F., Fokin, A., Kostin, S., Zimmermann, R., et al. (2000). Apoptosis is initiated by myocardial ischemia and executed during reperfusion. Journal of Molecular and Cellular Cardiology, 32, 197–208.
Lee, P., Sata, M., Lefer, D. J., Factor, S. M., Walsh, K., & Kitsis, R. N. (2003). Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia–reperfusion in vivo. American Journal of Physiology Heart and Circulatory Physiology, 284, H456–H463.
Chen, Z., Chua, C. C., Ho, Y. S., Hamdy, R. C., & Chua, B. H. (2001). Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. American Journal of Physiology Heart and Circulatory Physiology, 280, H2313–H2320.
Bialik, S., Cryns, V. L., Drincic, A., Miyata, S., Wollowick, A. L., Srinivasan, A., et al. (1999). The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circulation Research, 85, 403–414.
Gottlieb, R. A., Burleson, K. O., Kloner, R. A., Babior, B. M., & Engler, R. L. (1994). Reperfusion injury induces apoptosis in rabbit cardiomyocytes. Journal of Clinical Investigation, 94, 1621–1628.
Zhao, Z. Q., & Vinten-Johansen, J. (2002). Myocardial apoptosis and ischemic preconditioning. Cardiovascular Research, 55, 438–455.
Black, S. C., Huang, J. Q., Rezaiefar, P., Radinovic, S., Eberhart, A., Nicholson, D. W., et al. (1998). Co-localization of the cysteine protease caspase-3 with apoptotic myocytes after in vivo myocardial ischemia and reperfusion in the rat. Journal of Molecular and Cellular Cardiology, 30, 733–742.
Chakrabarti, S., Hoque, A. N., & Karmazyn, M. (1997). A rapid ischemia-induced apoptosis in isolated rat hearts and its attenuation by the sodium-hydrogen exchange inhibitor HOE 642 (cariporide). Journal of Molecular and Cellular Cardiology, 29, 3169–3174.
Borutaite, V., Budriunaite, A., Morkuniene, R., & Brown, G. C. (2001). Release of mitochondrial cytochrome c and activation of cytosolic caspases induced by myocardial ischaemia. Biochimica et Biophysica Acta, 1537, 101–109.
Borutaite, V., Jekabsone, A., Morkuniene, R., & Brown, G. C. (2003). Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. Journal of Molecular and Cellular Cardiology, 35, 357–366.
Akiyama, K., Gluckman, T. L., Terhakopian, A., Jinadasa, P. M., Narayan, S., Singaswamy, S., et al. (1997). Apoptosis in experimental myocardial infarction in situ and in the perfused heart in vitro. Tissue and Cell, 29, 733–743.
Zhao, Z. Q. (2004). Oxidative stress-elicited myocardial apoptosis during reperfusion. Current Opinion in Pharmacology, 4, 159–165.
Kagan, V. E., Tyurina, Y. Y., Bayir, H., Chu, C. T., Kapralov, A. A., Vlasova, I. I., et al. (2006). The “pro-apoptotic genies” get out of mitochondria: Oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chemico Biological Interactions, 163, 15–28.
St-Pierre, J., Brand, M. D., & Boutilier, R. G. (2000). Mitochondria as ATP consumers: Cellular treason in anoxia. Proceedings of the National Academy of Sciences of the United States of America, 97, 8670–8674.
Chen, Q., Moghaddas, S., Hoppel, C. L., & Lesnefsky, E. J. (2008). Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. American Journal of Physiology Cell Physiology, 294, C460–C466.
Andreyev, A. Y., Kushnareva, Y. E., & Starkov, A. A. (2005). Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc), 70, 200–214.
Ferrari, R., Ceconi, C., Curello, S., Cargnoni, A., De Giuli, F., & Visioli, O. (1992). Occurrence of oxidative stress during myocardial reperfusion. Molecular and Cellular Biochemistry, 111, 61–69.
Dhalla, N. S., Elmoselhi, A. B., Hata, T., & Makino, N. (2000). Status of myocardial antioxidants in ischemia–reperfusion injury. Cardiovascular Research, 47, 446–456.
Ferrari, R., Guardigli, G., Mele, D., Percoco, G. F., Ceconi, C., & Curello, S. (2004). Oxidative stress during myocardial ischaemia and heart failure. Current Pharmaceutical Design, 10, 1699–1711.
Powers, S. K., Murlasits, Z., Wu, M., & Kavazis, A. N. (2007). Ischemia–reperfusion-induced cardiac injury: A brief review. Medicine and Science in Sports and Exercise, 39, 1529–1536.
Halestrap, A. P. (2009). What is the mitochondrial permeability transition pore? Journal of Molecular and Cellular Cardiology, 46, 821–831.
Zamzami, N., Marchetti, P., Castedo, M., Hirsch, T., Susin, S. A., Masse, B., et al. (1996). Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Letters, 384, 53–57.
Skulachev, V. P. (2000). How proapoptotic proteins can escape from mitochondria? Free Radical Biology and Medicine, 29, 1056–1059.
Borutaite, V., & Brown, G. C. (2003). Mitochondria in apoptosis of ischemic heart. FEBS Letters, 541, 1–5.
Baines, C. P. (2009). The molecular composition of the mitochondrial permeability transition pore. Journal of Molecular and Cellular Cardiology, 46, 850–857.
Javadov, S., & Karmazyn, M. (2007). Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cellular Physiology and Biochemistry, 20, 1–22.
Kim, J. S., He, L., & Lemasters, J. J. (2003). Mitochondrial permeability transition: A common pathway to necrosis and apoptosis. Biochemical and Biophysical Research Communications, 304, 463–470.
Dedkova, E. N., & Blatter, L. A. (2008). Mitochondrial Ca2+ and the heart. Cell Calcium, 44, 77–91.
Dong, Z., Saikumar, P., Weinberg, J. M., & Venkatachalam, M. A. (2006). Calcium in cell injury and death. Annu Rev Pathol, 1, 405–434.
Chen, M., Won, D. J., Krajewski, S., & Gottlieb, R. A. (2002). Calpain and mitochondria in ischemia/reperfusion injury. The Journal of Biological Chemistry, 277, 29181–29186.
Novgorodov, S. A., & Gudz, T. I. (2009). Ceramide and mitochondria in ischemia/reperfusion. Journal of Cardiovascular Pharmacology, 53, 198–208.
Vaseva, A. V., & Moll, U. M. (2009). The mitochondrial p53 pathway. Biochimica et Biophysica Acta, 1787, 414–420.
Marchenko, N. D., Zaika, A., & Moll, U. M. (2000). Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. The Journal of Biological Chemistry, 275, 16202–16212.
Erster, S., Mihara, M., Kim, R. H., Petrenko, O., & Moll, U. M. (2004). In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Molecular and Cellular Biology, 24, 6728–6741.
Park, B. S., Song, Y. S., Yee, S. B., Lee, B. G., Seo, S. Y., Park, Y. C., et al. (2005). Phospho-ser 15–p53 translocates into mitochondria and interacts with Bcl-2 and Bcl-xL in eugenol-induced apoptosis. Apoptosis, 10, 193–200.
Mihara, M., Erster, S., Zaika, A., Petrenko, O., Chittenden, T., Pancoska, P., et al. (2003). p53 has a direct apoptogenic role at the mitochondria. Molecular Cell, 11, 577–590.
Webster, K. A., Discher, D. J., Kaiser, S., Hernandez, O., Sato, B., & Bishopric, N. H. (1999). Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. Journal of Clinical Investigation, 104, 239–252.
Mocanu, M. M., & Yellon, D. M. (2003). p53 down-regulation: A new molecular mechanism involved in ischaemic preconditioning. FEBS Letters, 555, 302–306.
Liu, P., Xu, B., Cavalieri, T. A., & Hock, C. E. (2008). Inhibition of p53 by pifithrin-alpha reduces myocyte apoptosis and leukocyte transmigration in aged rat hearts following 24 hours of reperfusion. Shock, 30, 545–551.
Toth, A., Jeffers, J. R., Nickson, P., Min, J. Y., Morgan, J. P., Zambetti, G. P., et al. (2006). Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia–reperfusion. American Journal of Physiology Heart and Circulatory Physiology, 291, H52–H60.
Adams, J. M., & Cory, S. (2007). Bcl-2-regulated apoptosis: Mechanism and therapeutic potential. Current Opinion in Immunology, 19, 488–496.
Chipuk, J. E., Maurer, U., Green, D. R., & Schuler, M. (2003). Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell, 4, 371–381.
Kumar, D., & Jugdutt, B. I. (2003). Apoptosis and oxidants in the heart. Journal of Laboratory and Clinical Medicine, 142, 288–297.
Kuwana, T., Mackey, M. R., Perkins, G., Ellisman, M. H., Latterich, M., Schneiter, R., et al. (2002). Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell, 111, 331–342.
Gustafsson, A. B., & Gottlieb, R. A. (2007). Bcl-2 family members and apoptosis, taken to heart. American Journal of Physiology Cell Physiology, 292, C45–C51.
Zhu, L., Yu, Y., Chua, B. H., Ho, Y. S., & Kuo, T. H. (2001). Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. Journal of Molecular and Cellular Cardiology, 33, 2135–2144.
Capano, M., & Crompton, M. (2006). Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochemical Journal, 395, 57–64.
Kirshenbaum, L. A., & de Moissac, D. (1997). The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation, 96, 1580–1585.
Brocheriou, V., Hagege, A. A., Oubenaissa, A., Lambert, M., Mallet, V. O., Duriez, M., et al. (2000). Cardiac functional improvement by a human Bcl-2 transgene in a mouse model of ischemia/reperfusion injury. The Journal of Gene Medicine, 2, 326–333.
Weisleder, N., Taffet, G. E., & Capetanaki, Y. (2004). Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America, 101, 769–774.
Grover, G. J., Marone, P. A., Koetzner, L., & Seto-Young, D. (2008). Energetic signalling in the control of mitochondrial F1F0 ATP synthase activity in health and disease. International Journal of Biochemistry and Cell Biology, 40, 2698–2701.
Classen, J. B., Mergner, W. J., & Costa, M. (1989). ATP hydrolysis by ischemic mitochondria. Journal of Cellular Physiology, 141, 53–59.
Imahashi, K., Schneider, M. D., Steenbergen, C., & Murphy, E. (2004). Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circulation Research, 95, 734–741.
Maulik, N., Engelman, R. M., Rousou, J. A., Flack, J. E., 3rd, Deaton, D., & Das, D. K. (1999). Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation, 100, II369–II375.
Hattori, R., Hernandez, T. E., Zhu, L., Maulik, N., Otani, H., Kaneda, Y., et al. (2001). An essential role of the antioxidant gene Bcl-2 in myocardial adaptation to ischemia: an insight with antisense Bcl-2 therapy. Antioxidants & Redox Signaling, 3, 403–413.
Wallace, D. C., Shoffner, J. M., Trounce, I., Brown, M. D., Ballinger, S. W., Corral-Debrinski, M., et al. (1995). Mitochondrial DNA mutations in human degenerative diseases and aging. Biochimica et Biophysica Acta, 1271, 141–151.
Yasuda, T., Kamiya, H., Tanaka, Y., & Watanabe, G. (2001). Ultra-short-acting cardioselective beta-blockade attenuates postischemic cardiac dysfunction in the isolated rat heart. European Journal of Cardio-Thoracic Surgery, 19, 647–652.
Sabbah, H. N. (1999). The cellular and physiologic effects of beta blockers in heart failure. Clinical Cardiology, 22(Suppl 5), V16–V20.
Kawai, K., Qin, F., Shite, J., Mao, W., Fukuoka, S., & Liang, C. S. (2004). Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. American Journal of Physiology Heart and Circulatory Physiology, 287, H1003–H1012.
Nayler, W. G., Ferrari, R., & Williams, A. (1980). Protective effect of pretreatment with verapamil, nifedipine and propranolol on mitochondrial function in the ischemic and reperfused myocardium. American Journal of Cardiology, 46, 242–248.
Vandeplassche, G., Lu, H. R., Wouters, L., Flameng, W., & Borgers, M. (1991). Normothermic ischemic cardiac arrest in the isolated working rabbit heart: effects of dl-nebivolol and atenolol. Basic Research in Cardiology, 86, 21–31.
Carreira, R. S., Monteiro, P., Gon Alves, L. M., & Providencia, L. A. (2006). Carvedilol: Just another Beta-blocker or a powerful cardioprotector? Cardiovascular & Hematological Disorders Drug Targets, 6, 257–266.
Oliveira, P. J., Goncalves, L., Monteiro, P., Providencia, L. A., & Moreno, A. J. (2005). Are the antioxidant properties of carvedilol important for the protection of cardiac mitochondria? Current Vascular Pharmacology, 3, 147–158.
Yue, T. L., Cheng, H. Y., Lysko, P. G., McKenna, P. J., Feuerstein, R., Gu, J. L., et al. (1992). Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. Journal of Pharmacology and Experimental Therapeutics, 263, 92–98.
Yue, T. L., McKenna, P. J., Ruffolo, R. R., Jr., & Feuerstein, G. (1992). Carvedilol, a new beta-adrenoceptor antagonist and vasodilator antihypertensive drug, inhibits superoxide release from human neutrophils. European Journal of Pharmacology, 214, 277–280.
Spallarossa, P., Garibaldi, S., Altieri, P., Fabbi, P., Manca, V., Nasti, S., et al. (2004). Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. Journal of Molecular and Cellular Cardiology, 37, 837–846.
Feuerstein, G., Yue, T. L., Ma, X., & Ruffolo, R. R. (1998). Novel mechanisms in the treatment of heart failure: Inhibition of oxygen radicals and apoptosis by carvedilol. Progress in Cardiovascular Diseases, 41, 17–24.
Oliveira, P. J., Esteves, T., Rolo, A. P., Palmeira, C. M., & Moreno, A. J. (2004). Carvedilol inhibits the mitochondrial permeability transition by an antioxidant mechanism. Cardiovascular Toxicology, 4, 11–20.
Oliveira, P. J., Bjork, J. A., Santos, M. S., Leino, R. L., Froberg, M. K., Moreno, A. J., et al. (2004). Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicology and Applied Pharmacology, 200, 159–168.
Sgobbo, P., Pacelli, C., Grattagliano, I., Villani, G., & Cocco, T. (2007). Carvedilol inhibits mitochondrial complex I and induces resistance to H2O2 -mediated oxidative insult in H9C2 myocardial cells. Biochimica et Biophysica Acta, 1767, 222–232.
Noguchi, N., Nishino, K., & Niki, E. (2000). Antioxidant action of the antihypertensive drug, carvedilol, against lipid peroxidation. Biochemical Pharmacology, 59, 1069–1076.
Oliveira, P. J., Coxito, P. M., Rolo, A. P., Santos, D. L., Palmeira, C. M., & Moreno, A. J. (2001). Inhibitory effect of carvedilol in the high-conductance state of the mitochondrial permeability transition pore. European Journal of Pharmacology, 412, 231–237.
Oliveira, P. J., Rolo, A. P., Palmeira, C. M., & Moreno, A. J. (2001). Carvedilol reduces mitochondrial damage induced by hypoxanthine/xanthine oxidase: Relevance to hypoxia/reoxygenation injury. Cardiovascular Toxicology, 1, 205–213.
Oliveira, P. J., Santos, D. J., & Moreno, A. J. (2000). Carvedilol inhibits the exogenous NADH dehydrogenase in rat heart mitochondria. Archives of Biochemistry and Biophysics, 374, 279–285.
Nohl, H. (1987). Demonstration of the existence of an organo-specific NADH dehydrogenase in heart mitochondria. European Journal of Biochemistry, 169, 585–591.
Oliveira, P. J., Marques, M. P., Batista de Carvalho, L. A., & Moreno, A. J. (2000). Effects of carvedilol on isolated heart mitochondria: Evidence for a protonophoretic mechanism. Biochemical and Biophysical Research Communications, 276, 82–87.
Kristian, T., & Siesjo, B. K. (1998). Calcium in ischemic cell death. Stroke, 29, 705–718.
Ferrari, R., & Visioli, O. (1991). Calcium channel blockers and ischaemic heart disease: Theoretical expectations and clinical experience. European Heart Journal, 12(Suppl F), 18–24.
Mohan, I. K., Khan, M., Wisel, S., Selvendiran, K., Sridhar, A., Carnes, C. A., et al. (2009). Cardioprotection by HO-4038, a novel verapamil derivative, targeted against ischemia and reperfusion-mediated acute myocardial infarction. American journal of Physiology Heart and Circulatory Physiology, 296, H140–H151.
Matlib, M. A., & McFarland, K. L. (1991). Diltiazem inhibition of sodium-induced calcium release. Effects on energy metabolism of heart mitochondria. American Journal of Hypertension, 4, 435S–441S.
Tabouy, L., Chauvet-Monges, A. M., Salducci, M. D., & Crevat, A. (1994). Protective effect of some calcium antagonists against mitochondrial calcium injury. International Journal of Tissue Reactions, 16, 221–228.
Vavrinkova, H., Tutterova, M., Stopka, P., Divisova, J., Kazdova, L., & Drahota, Z. (2001). The effect of captopril on nitric oxide formation and on generation of radical forms of mitochondrial respiratory chain compounds in ischemic rat heart. Physiological Research, 50, 481–489.
Yanagishita, T., Tomita, M., Itoh, S., Mukae, S., Arata, H., Ishioka, H., et al. (1997). Protective effect of captopril on ischemic myocardium. Japanese Circulation Journal, 61, 161–169.
Sanbe, A., Tanonaka, K., Kobayasi, R., & Takeo, S. (1995). Effects of long-term therapy with ACE inhibitors, captopril, enalapril and trandolapril, on myocardial energy metabolism in rats with heart failure following myocardial infarction. Journal of Molecular and Cellular Cardiology, 27, 2209–2222.
Monteiro, P., Oliveira, P. J., Concalves, L., & Providencia, L. A. (2003). Pharmacological modulation of mitochondrial function during ischemia and reperfusion. Revista Portuguesa de Cardiologia, 22, 407–429.
Swedberg, K., Held, P., Kjekshus, J., Rasmussen, K., Ryden, L., & Wedel, H. (1992). Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). New England Journal of Medicine, 327, 678–684.
Monteiro, P., Duarte, A. I., Goncalves, L. M., & Providencia, L. A. (2005). Valsartan improves mitochondrial function in hearts submitted to acute ischemia. European Journal of Pharmacology, 518, 158–164.
Singh, B. N. (2006). Amiodarone: A multifaceted antiarrhythmic drug. Current Cardiology Reports, 8, 349–355.
Varbiro, G., Toth, A., Tapodi, A., Veres, B., Sumegi, B., & Gallyas, F., Jr. (2003). Concentration dependent mitochondrial effect of amiodarone. Biochemical Pharmacology, 65, 1115–1128.
Spedding, M., Tillement, J. P., Morin, D., & Le Ridant, A. (1999). Medicines interacting with mitochondria: Anti-ischemic effects of trimetazidine. Therapie, 54, 627–635.
Kantor, P. F., Lucien, A., Kozak, R., & Lopaschuk, G. D. (2000). The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circulation Research, 86, 580–588.
Grynberg, A., & Demaison, L. (1996). Fatty acid oxidation in the heart. Journal of Cardiovascular Pharmacology, 28(Suppl 1), S11–S17.
Morin, D., Hauet, T., Spedding, M., & Tillement, J. (2001). Mitochondria as target for antiischemic drugs. Advanced Drug Delivery Reviews, 49, 151–174.
Guarnieri, C., Finelli, C., Zini, M., & Muscari, C. (1997). Effects of trimetazidine on the calcium transport and oxidative phosphorylation of isolated rat heart mitochondria. Basic Research in Cardiology, 92, 90–95.
Veitch, K., Maisin, L., & Hue, L. (1995). Trimetazidine effects on the damage to mitochondrial functions caused by ischemia and reperfusion. American Journal of Cardiology, 76, 25B–30B.
Salloum, F. N., Ockaili, R. A., Wittkamp, M., Marwaha, V. R., & Kukreja, R. C. (2006). Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. Journal of Molecular and Cellular Cardiology, 40, 405–411.
Kukreja, R. C., Salloum, F., Das, A., Ockaili, R., Yin, C., Bremer, Y. A., et al. (2005). Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascular Pharmacology, 42, 219–232.
Salloum, F. N., Takenoshita, Y., Ockaili, R. A., Daoud, V. P., Chou, E., Yoshida, K., et al. (2007). Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. Journal of Molecular and Cellular Cardiology, 42, 453–458.
Fernandes, M. A., Marques, R. J., Vicente, J. A., Santos, M. S., Monteiro, P., Moreno, A. J., et al. (2008). Sildenafil citrate concentrations not affecting oxidative phosphorylation depress H2O2 generation by rat heart mitochondria. Molecular and Cellular Biochemistry, 309, 77–85.
Wang, X., Fisher, P. W., Xi, L., & Kukreja, R. C. (2008). Essential role of mitochondrial Ca2+ -activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. Journal of Molecular and Cellular Cardiology, 44, 105–113.
Reffelmann, T., & Kloner, R. A. (2005). Pharmacotherapy of erectile dysfunction: Focus on cardiovascular safety. Expert Opinion on Drug Safety, 4, 531–540.
Garlid, K. D., Paucek, P., Yarov-Yarovoy, V., Murray, H. N., Darbenzio, R. B., D’Alonzo, A. J., et al. (1997). Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circulation Research, 81, 1072–1082.
Baines, C. P., Liu, G. S., Birincioglu, M., Critz, S. D., Cohen, M. V., & Downey, J. M. (1999). Ischemic preconditioning depends on interaction between mitochondrial KATP channels and actin cytoskeleton. American Journal of Physiology, 276, H1361–H1368.
Takashi, E., Wang, Y., & Ashraf, M. (1999). Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circulation Research, 85, 1146–1153.
Pasdois, P., Beauvoit, B., Tariosse, L., Vinassa, B., Bonoron-Adele, S., & Dos Santos, P. (2008). Effect of diazoxide on flavoprotein oxidation and reactive oxygen species generation during ischemia–reperfusion: A study on Langendorff-perfused rat hearts using optic fibers. American Journal of Physiology Heart and Circulatory Physiology, 294, H2088–H2097.
Ohnuma, Y., Miura, T., Miki, T., Tanno, M., Kuno, A., Tsuchida, A., et al. (2002). Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. American Journal of Physiology Heart and Circulatory Physiology, 283, H440–H447.
Korge, P., Honda, H. M., & Weiss, J. N. (2002). Protection of cardiac mitochondria by diazoxide and protein kinase C: Implications for ischemic preconditioning. Proceedings of the National Academy of Sciences of the United States of America, 99, 3312–3317.
Minatoguchi, S., Wang, N., Uno, Y., Arai, M., Hashimoto, K., Hashimoto, Y., et al. (2001). Combination of miglitol, an anti-diabetic drug, and nicorandil markedly reduces myocardial infarct size through opening the mitochondrial K(ATP) channels in rabbits. British Journal of Pharmacology, 133, 1041–1046.
Ozcan, C., Bienengraeber, M., Dzeja, P. P., & Terzic, A. (2002). Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. American Journal of Physiology Heart and Circulatory Physiology, 282, H531–H539.
Iwai, T., Tanonaka, K., Motegi, K., Inoue, R., Kasahara, S., & Takeo, S. (2002). Nicorandil preserves mitochondrial function during ischemia in perfused rat heart. European Journal of Pharmacology, 446, 119–127.
Sato, T., Sasaki, N., O’Rourke, B., & Marban, E. (2000). Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. Journal of the American College of Cardiology, 35, 514–518.
Halestrap, A. P., & Pasdois, P. (2009). The role of the mitochondrial permeability transition pore in heart disease. Biochimica et Biophysica Acta, 1787, 1402–1415.
Halestrap, A. P., & Davidson, A. M. (1990). Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis–trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochemical Journal, 268, 153–160.
Halestrap, A. P., Clarke, S. J., & Javadov, S. A. (2004). Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovascular Research, 61, 372–385.
Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., et al. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature, 434, 658–662.
Nakagawa, T., Shimizu, S., Watanabe, T., Yamaguchi, O., Otsu, K., Yamagata, H., et al. (2005). Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature, 434, 652–658.
Halestrap, A. P., Connern, C. P., Griffiths, E. J., & Kerr, P. M. (1997). Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Molecular and Cellular Biochemistry, 174, 167–172.
Magnasco, A., Rossi, A., Catarsi, P., Gusmano, R., Ginevri, F., Perfumo, F., et al. (2008). Cyclosporin and organ specific toxicity: Clinical aspects, pharmacogenetics and perspectives. Current Clinical Pharmacology, 3, 166–173.
Ferrari, R., Merli, E., Cicchitelli, G., Mele, D., Fucili, A., & Ceconi, C. (2004). Therapeutic effects of l-carnitine and propionyl-l-carnitine on cardiovascular diseases: A review. Annals of the New York Academy of Sciences, 1033, 79–91.
Andrieu-Abadie, N., Jaffrezou, J. P., Hatem, S., Laurent, G., Levade, T., & Mercadier, J. J. (1999). l-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: Role of inhibition of ceramide generation. Faseb Journal, 13, 1501–1510.
Mutomba, M. C., Yuan, H., Konyavko, M., Adachi, S., Yokoyama, C. B., Esser, V., et al. (2000). Regulation of the activity of caspases by l-carnitine and palmitoylcarnitine. FEBS Letters, 478, 19–25.
Gao, L., Laude, K., & Cai, H. (2008). Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Veterinary Clinics of North America Small Animal Practice, 38, 137–155.
Yamawaki, M., Sasaki, N., Shimoyama, M., Miake, J., Ogino, K., Igawa, O., et al. (2004). Protective effect of edaravone against hypoxia-reoxygenation injury in rabbit cardiomyocytes. British Journal of Pharmacology, 142, 618–626.
Watanabe, T., Yuki, S., Egawa, M., & Nishi, H. (1994). Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. Journal of Pharmacology and Experimental Therapeutics, 268, 1597–1604.
Rajesh, K. G., Sasaguri, S., Suzuki, R., & Maeda, H. (2003). Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. American Journal of Physiology Heart and Circulatory Physiology, 285, H2171–H2178.
Yamazaki, K., Miwa, S., Toyokuni, S., Nemoto, S., Oriyanhan, W., Takaba, K., et al. (2009). Effect of edaravone, a novel free radical scavenger, supplemented to cardioplegia on myocardial function after cardioplegic arrest: in vitro study of isolated rat heart. Heart and Vessels, 24, 228–235.
Mallet, R. T., Sun, J., Knott, E. M., Sharma, A. B., & Olivencia-Yurvati, A. H. (2005). Metabolic cardioprotection by pyruvate: Recent progress. Experimental Biology and Medicine (Maywood), 230, 435–443.
Mallet, R. T., & Sun, J. (1999). Mitochondrial metabolism of pyruvate is required for its enhancement of cardiac function and energetics. Cardiovascular Research, 42, 149–161.
Kerr, P. M., Suleiman, M. S., & Halestrap, A. P. (1999). Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. American Journal of Physiology, 276, H496–H502.
Hamilton, K. L. (2007). Antioxidants and cardioprotection. Medicine and Science in Sports and Exercise, 39, 1544–1553.
Pain, T., Yang, X. M., Critz, S. D., Yue, Y., Nakano, A., Liu, G. S., et al. (2000). Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circulation Research, 87, 460–466.
Adlam, V. J., Harrison, J. C., Porteous, C. M., James, A. M., Smith, R. A., Murphy, M. P., et al. (2005). Targeting an antioxidant to mitochondria decreases cardiac ischemia–reperfusion injury. Faseb Journal, 19, 1088–1095.
Bakeeva, L. E., Barskov, I. V., Egorov, M. V., Isaev, N. K., Kapelko, V. I., Kazachenko, A. V., et al. (2008). Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry (Mosc), 73, 1288–1299.
Fink, M. P., Macias, C. A., Xiao, J., Tyurina, Y. Y., Delude, R. L., Greenberger, J. S., et al. (2007). Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants. Critical Care Medicine, 35, S461–S467.
James, A. M., Cocheme, H. M., & Murphy, M. P. (2005). Mitochondria-targeted redox probes as tools in the study of oxidative damage and ageing. Mechanisms of Ageing and Development, 126, 982–986.
Giacomo, C. G., & Antonio, M. (2007). Melatonin in cardiac ischemia/reperfusion-induced mitochondrial adaptive changes. Cardiovascular Hematological Disorders Drug Targets, 7, 163–169.
Acuna-Castroviejo, D., Escames, G., Rodriguez, M. I., & Lopez, L. C. (2007). Melatonin role in the mitochondrial function. Front Bioscience, 12, 947–963.
Ceyran, H., Narin, F., Narin, N., Akgun, H., Ceyran, A. B., Ozturk, F., et al. (2008). The effect of high dose melatonin on cardiac ischemia- reperfusion injury. Yonsei Medical Journal, 49, 735–741.
Petrosillo, G., Di Venosa, N., Pistolese, M., Casanova, G., Tiravanti, E., Colantuono, G., et al. (2006). Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia- reperfusion: Role of cardiolipin. Faseb Journal, 20, 269–276.
Ostadal, B., Netuka, I., Maly, J., Besik, J., & Ostadalova, I. (2009). Gender differences in cardiac ischemic injury and protection—experimental aspects. Experimental Biology and Medicine (Maywood), 234, 1011–1019.
Booth, E. A., & Lucchesi, B. R. (2008). Estrogen-mediated protection in myocardial ischemia–reperfusion injury. Cardiovascular Toxicology, 8, 101–113.
Xu, Y., Arenas, I. A., Armstrong, S. J., Plahta, W. C., Xu, H., & Davidge, S. T. (2006). Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-alpha. Cardiovascular Research, 69, 836–844.
Duckles, S. P., Krause, D. N., Stirone, C., & Procaccio, V. (2006). Estrogen and mitochondria: A new paradigm for vascular protection? Molecular Interventions, 6, 26–35.
Morkuniene, R., Jekabsone, A., & Borutaite, V. (2002). Estrogens prevent calcium-induced release of cytochrome c from heart mitochondria. FEBS Letters, 521, 53–56.
Yang, S. H., Liu, R., Perez, E. J., Wen, Y., Stevens, S. M., Jr., Valencia, T., et al. (2004). Mitochondrial localization of estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America, 101, 4130–4135.
Morkuniene, R., Arandarcikaite, O., & Borutaite, V. (2006). Estradiol prevents release of cytochrome c from mitochondria and inhibits ischemia-induced apoptosis in perfused heart. Experimental Gerontology, 41, 704–708.
Sugishita, K., Li, F., Su, Z., & Barry, W. H. (2003). Anti-oxidant effects of estrogen reduce [Ca2+]i during metabolic inhibition. Journal of Molecular and Cellular Cardiology, 35, 331–336.
Wang, Q. D., Pernow, J., Sjoquist, P. O., & Ryden, L. (2002). Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovascular Research, 55, 25–37.
Berthiaume, J. M., Oliveira, P. J., Fariss, M. W., & Wallace, K. B. (2005). Dietary vitamin E decreases doxorubicin-induced oxidative stress without preventing mitochondrial dysfunction. Cardiovascular Toxicology, 5, 257–267.
Acknowledgments
The work in the authors’ laboratory is supported by the Portuguese Foundation for Science and Technology (SFRH/BD/31655/2006 to Marco G. Alves, POCI/SAU-OBS/55802/2004 to Rui A. Carvalho).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machado, N.G., Alves, M.G., Carvalho, R.A. et al. Mitochondrial Involvement in Cardiac Apoptosis During Ischemia and Reperfusion: Can We Close the Box?. Cardiovasc Toxicol 9, 211–227 (2009). https://doi.org/10.1007/s12012-009-9055-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-009-9055-1