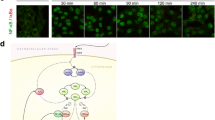
Abstract
In the article, we discuss the state of art and perspectives in deterministic and stochastic models of NFκB regulatory module. The NFκB is a transcription factor controlling various immune responses including inflammation and apoptosis. It is tightly regulated by at least two negative feedback loops involving IκBα and A20. This mode of regulation results in nucleus-to-cytoplasm oscillations in NFκB localization, which induce subsequent waves of NFκB responsive genes. Single cell experiments carried by several groups provided comprehensive evidence that stochastic effects play an important role in NFκB regulation. From modeling point of view, living cells might be considered noisy or stochastic biochemical reactors. In eukaryotic cells, in which the number of protein or mRNA molecules is relatively large, stochastic effects primarily originate in regulation of gene activity. Transcriptional activity of a gene can be initiated by trans-activator molecules binding to the specific regulatory site(s) in the target gene. The stochastic event of gene activation is amplified by transcription and translation, since it results in a burst of mRNA molecules, and each copy of mRNA then serves as a template for numerous protein molecules. Another potential source of variability can be receptors activation. At low-dose stimulation, important in cell-to-cell signaling, the number of active receptors can be low enough to introduce substantial noise to downstream signaling. Stochastic modeling confirms the large variability in cell responses and shows that no cell behaves like an “average” cell. This high cell-to-cell variability can be one of the weapons of the immune defense. Such non-deterministic defense may be harder to overcome by relatively simple programs coded in viruses and other pathogens.








Similar content being viewed by others
References
Gillespie, D. T. (1977). Exact stochastic simulations of coupled chemical reactions. The Journal of Physical Chemistry, 81, 2340–2361.
Haseltine, E. L., & Rawlings, J. B. (2002). Approximate simulation of coupled fast and slow reactions for stochastic chemical kinetics. The Journal of Chemical Physics, 117, 6959–6969.
Brasier, A. R. (2006). The NF-κB regulatory networks. Cardiovascular Toxicology, 6, 111–130.
Hoffmann, A., & Baltimore, D. (2006). Circuitry of nuclear κB factor signaling. Immunological Review, 210, 171–186.
Sun, S.-C., Ganchi, P. A., Ballard, D. W., & Greene, W. C. (1993). NF-κB controls expression of inhibitor IκBα: Evidence for an inducible autoregulatory pathway. Science, 259, 1912–1915.
Krikos, A., Laherty, C. D., & Dixit, V. M. (1992). Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. The Journal of Biological Chemistry, 267, 17971–17976.
Lee, E. G., Boone, D. L., Chai, S., Libby, S. L., Chien, M., Lodolce, J. P., & Ma, A. (2000). Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science, 289, 2350–2354.
Bauch, A., & Superti-Furga, G. (2006). Charting protein complexes, signaling pathways, and networks in the immune system. Immunological Reviews, 210, 187–207.
Karin, M. (1999). The beginning of the end: IκB kinase (IKK) and NF-κB activation. The Journal of Biological Chemistry, 274, 27339–27342.
Hoffmann, A., Levchenko, A. Scott, M. L., & Baltimore, D. (2002). The IκB − NF − κB signaling module: Temporal control and selective gene activation. Science, 298, 1241–1245.
Kearns, J. D., Basak, S., Werner, Sh. L., Huang, Ch. H., & Hoffmann, A. (2006). IκBɛ provides negative feedback to control NF-κB oscillations, signaling dynamics, and inflammatory gene expression. The Journal of Cell Biology DOI: 10.1083/jcb.200510155 JCB
Lipniacki, T., Paszek, P., Brasier, A. R., Luxon, B., & Kimmel, M. (2004). Mathematical model of NF-κB regulatory module. Journal of Theoretical Biology, 228, 195–215.
Park, S. G., Lee, T., Kang, H. Y., Park, K., Cho, K-H., & Jung, G. (2006). The influence of the signal dynamics of activated form of IKK on NFκB and anti-apoptotic gene expression: A systems biology approach. FEBS Letters, 580, 822–830.
Ko, M. S. H. (1991). Stochastic model for gene induction. Journal of Theoretical Biology, 153, 181–194.
McAdams, H. H., & Arkin, A. (1997). Stochastic mechanisms in gene expression. Proceedings of the National Academy of Sciences USA, 94, 814–819.
Kierzek, A. M., Zaim, J., & Zielenkiewicz, P. (2001). The effect of transcription and translation initiation frequencies on the stochastic fluctuations in prokaryotic gene expression. The Journal of Biological Chemistry, 276, 8165–8172.
Thattai, M., & Oudenaarden, A. (2001). Intrinsic noise in gene regulatory networks. Proceedings of the National Academy of Sciences USA, 98, 8614–8619.
Tomioka, R., Kimura, H., Kobayashi, T. J., & Aihara, K. (2004). Multivariate analysis of noise in genetic regulatory networks. Journal of Theoretical Biology, 229, 501–521.
Kærn, M., Elston, T. C., Blake, W. J., & Collins, J. J. (2005). Stochasticity in gene expression from theories to phenotypes. Nature Reviews, 6, 451–464.
Walters, M. C., Fiering, S., Eidemiller, J., Magis, W., Groudine, M., & Martin, D. I. K. (1995). Enhancers increase the probability but not the level of gene expression. Proceedings of the National Academy of Sciences USA, 92, 7125–7129.
Blake, W. J., Kærn, M., Cantor, C. R., & Collins, J. J. (2003). Noise in eucaryotic gene expression. Nature, 422, 633–637.
Takasuka, N., White, M. R. H., Wood, C. D., Robertson, W. R., & Davis, J. R. E. (1998). Dynamic changes in prolactin promoter activation in individual living lactotrophic cells. Endocrinology, 139, 1361–1368.
Stirland, J. A., Seymour, Z. C., Windeatt, S., Norris, A. J., Stanley, P., Castro, M. G., Loudon, A. S. I., White, M. R. H., & Davis, J. R. E. (2003). Real-time imaging of gene promoter activity using an adenoviral reporter construct demonstrates transcriptional dynamics in normal anterior pituary cells. The Journal of Endocrinology 178, 61–69.
Raser, J. M., & O’Shea, E. K. (2004). Control of stochasticity in eukaryotic gene expression. Science, 304, 1811–1814.
Elowitz, M. B., Levine, A. J., Siggia, E. D., & Swain, P. S. (2002). Stochastic gene expression in a single cell. Science, 297, 1183–1186.
Kepler, T. B., & Elston, T. C. (2001). Stochasticity in transcriptional regulation: Origins, consequences, and mathematical representations. Biophysical Journal, 81, 3116–3136.
Pirone, J. R., & Elston, T. C. (2004). Fluctuations in transcription factor binding can be explain the graded and binary responses observed in inducible gene expression. Journal of Theoretical Biology, 226, 111–121.
Lipniacki, T., Paszek, P., Marciniak-Czochra, A., Brasier, A. R., & Kimmel, M. (2006). Transcriptional stochasticity in gene expression. Journal of Theoretical Biology, 238, 348–367.
van Kampen, N. G. (1992). Stochastic processes in chemistry and physics. Amsterdam: North-Holland.
Cao, Y., Petzold, L. R., Rathinam, M., Gillespie, D. T. (2004). The numerical stability of leaping methods for stochastic simulation of chemically reacting systems. The Journal of Chemical Physics, 121, 12169–12178.
Gillespie, D. T. (2001). Approximate accelerated stochastic simulation of chemically reacting system. The Journal of Physical Chemistry, 115, 1716–1733.
Rao, Ch. V., Arkin, A. P. (2003). Stochastic chemical kinetics and the quasi-steady-state assumption: Application to the Gillespie algorithm. The Journal of Chemical Physics, 118, 4999–5010.
Cao, Y., Gillespie, D. T., & Petzold, L. R. (2005). The slow-scale stochastic algorithm. The Journal of Chemical Physics, 122, 014116.
Puchalka, J., & Kierzek, A. M., (2004). Bridging the gap between stochastic and deterministic regimes in the kinetic simulations of the biochemical reaction networks. Biophysical Journal, 86, 1357–1372.
Nelson, D. E., Ihekwaba, A. E. C., Elliot, M., Johnson, J. R., Gibney, C. A., Foreman, B. E., et al. (2004). Oscillations in NF-κB signaling control the dynamics of gene expression. Science, 306, 704–708.
Carlotti, F., Chapman, R., Dower, S. K., & Qwarnstrom, E. E. (1999). Activation of nuclear factor κB in single living cells. The Journal of Biological Chemistry, 274, 37941–37949.
Nelson, G., Paraoan, L., Spiller, D. G., Wilde, G. J. C., Browne, A. M., & Djali, P. K., et al. (2002). Multi-parameter analysis of the kinetics of NF-κB signalling and transcription in single living cells. Journal of Cell Science, 115, 1137–1148.
Yang, L., Ross, K., & Qwarnstrom, E. E. (2003). RelA Control of IκBα phosphorylation. The Journal of Biological Chemistry, 278, 30881–30888.
Schooley, K., Zhu, P., Dower, S. K., & Qwarnstrom, E. E. (2003). Regulation of nuclear translocation of nuclear factor-κB RelA: Evidence for complex dynamics at the single-cell level. The Biochemical Journal, 369, 331–339.
Mendes, P. (2001). Modeling large biological systems from functional genomic data: Parameter estimation. In H. Kitano (Ed.), Foundations of systems biology (pp. 163–181). MIT Press.
Mendes, P. (1993). GEPASI: A software package for modelling the dynamics, steady states and control of biochemical and other systems. Computer Applications in the Biosciences, 9, 563–571; Mendes, P. (1997). Biochemistry by numbers: Simulation of biochemical pathways with Gepasi 3. Trends in Biochemical Sciences, 22, 361–363; Mendes, P., & Kell, D. B. (1998). Non-linear optimization of biochemical pathways: Applications to metabolic engineering and parameter estimation. Bioinformatics, 14, 869–883.
Fujarewicz, K., Kimmel, M., Lipniacki, T., & Świerniak, A. (2007). Adjoint systems for models of cell signaling pathways and their application to parameter fitting. IEEE/ACM Transactions on Computational Biology and Bioinformatics, 4, 322–335.
Werner, Sh. L., Barken, D., Hoffmann, A. (2005). Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science, 309, 1857–1861.
Covert, M. W., Leung, Th. H., Gaston, J. E., & Baltimore, D. (2005). Achieving stability of lipopolysaccharide-induced NFκB activation. Science, 309, 1854–1857.
Cheong, R., Bergmann, A., Werner, Sh. L., Regal, J., Hoffmann, A., & Levchenko, A. (2006). Transient IκB kinase activity mediates temporal NFκB dynamics in response to wide rage of tumor necrosis factor-α doses. The Journal of Biological Chemistry, 281, 2945–2950.
Gerondakis, S., Grossmann, M., Nakamura, Y., Pohl, T., & Grumont, R. (1999). Genetic approaches in mice to understand Rel/ NF-κB and IκB function: Transgenics and knockouts. Oncogene, 18, 6888–6895.
Jiang, X., Takahashi, N., Matsui, N., Tetsuka, T., & Okamoto, T (2003). The NF-κB activation in lymphotoxin receptor signaling depends on the phosphorylation of p65 at serine 536. The Journal of Biological Chemistry, 278, 919–926.
Cho, K.-H., Shin, S.-Y., Lee, H.-W., & Wolkenhauer, O. (2003). Investigations into the analysis and modeling of the TNFα-mediated NFκB-signaling pathway. Genome Research, 13, 2413–2422.
Delhase, M., Hayakawa, M., Chen, Y., & Karin, M. (1999). Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science, 284, 309–313.
Lipniacki, T., Paszek, P., Brasier, A. R., Luxon, B., & Kimmel. M. (2006). Stochastic regulations in early immune response. The Biophysical Journal, 90, 725–742.
Tian, B., Nowak, D. E., Jamaluddin, M., Wang, S., & Brasier, A. R. (2005). Identification of direct genomic targets downstream of the NF-kappa B transcription factor mediating TNFα signaling. The Journal of Biological Chemistry, 280, 17435–17448.
Paszek, P., Lipniacki, T., Brasier, A. R., Tian, B., Novak, D. E., & Kimmel, M. (2005). Stochastic effects of multiple regulators on expression profiles in Eukaryotes. The Journal of Theoretical Biology, 233, 423–433.
Femino, A. M, Fay, F. S., Fogarty, K., & Singer, R. H. (1998). Visualization of single RNA transcripts in situ. Science, 280, 585–590.
Carlotti, F, Dower, S. K., & Qwarnstrom, E. E. (2000). Dynamic shuttling of nuclear factor kappa B between the nucleus and cytoplasm as a consequence of inhibitor dissociation. The Journal of biological chemistry, 275, 41028–41034.
Nelson, D. E., Horton, C. A., See, V., Johnson, J. R., Nelson, G., Spiller, D. G., Kell D. B., & White, M. R. H. (2005). Response to comment on “Oscillations in NF-κB signaling control the dynamics of gene expression”. Science, 308, 52b.
Barken, D., Wang, Ch. J., Kearns, J., Cheong, R., Hoffmann, A., & Levchenko, A. (2005). Comment on “Oscillations in NF-κB signaling control the dynamics of gene expression”. Science, 308, 52a.
Hayot, F., & Jayaprakash, C. (2006). NFκB oscillations and cell-to-cell variability. The Journal of Theoretical Biology, 240, 583–591.
Jamaluddin, M., Wang, S., Boldogh, I., Tian, B., & Brasier, A. R. (2007). TNF-α-induced NF-κB/Rel A Ser (276) phosphorylation and enhance some formation on the IL-8 promoter is mediated by an ROS-dependent PKAc pathway. Cellular Signalling, 19, 1419–1433.
Tian, B., Nowak, D., & Brasier, A. R. (2005). A TNF induced gene expression program under oscillatory NF-κB control. BMC Genomics, 6, 137.
Acknowledgments
The authors would like to thank Drs. Allan R. Brasier and Michel R. H. White for discussion and Dr. Pawel Paszek for help with preparing figures. This work was supported by Polish Committee for Scientific Research Grants No. 4 T07A 001 30 and 3 T11A 019 29, and by NHLBI contract N01-HV-28184, Proteomic technologies in airway inflammation (A. Kurosky, P.I.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lipniacki, T., Kimmel, M. Deterministic and Stochastic Models of NFκB Pathway. Cardiovasc Toxicol 7, 215–234 (2007). https://doi.org/10.1007/s12012-007-9003-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-007-9003-x