Abstract
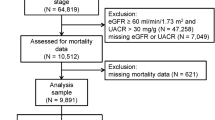
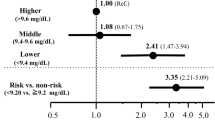
The association between selenium (Se) and lipid profile has been controversial in different populations, and the aim of the study was to investigate the relationship between Se and lipid profile in patients with chronic kidney disease (CKD). A total of 861 US adult patients with CKD (male: female = 404:457) from the National Health and Nutrition Examination Survey database were enrolled in this cross-sectional study. We used smoothing spline plots and multivariate binary logistic regression analyses to elucidate the relationships between blood Se and lipid profile. Multivariate adjusted smoothing spline plots showed that higher levels of blood Se were associated with higher levels of serum remnant cholesterol (RC), total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) levels. Threshold and saturation effects were also observed between serum RC, TC, TG, LDL-C, and blood Se. In multivariate binary logistic regression analyses, the fully adjusted model showed that as blood Se increases by every 1 µg/L, the OR of high RC, high TG and high LDL-C in patients was 1.012 (95% CI: 1.001, 1.023 P = 0.046), 1.011 (95% CI: 1.001, 1.021 P = 0.043) and 1.009 (95% CI: 1.003, 1.016 P = 0.012), respectively. Furthermore, stratified analyses showed that the associations between blood Se and high RC/high TG were significantly stronger in patients aged < 65 years. Higher levels of blood Se were associated with increased serum lipid profile levels and increased risk of high RC, high TC, high LDL-C, and low HDL-C dyslipidemia in adult patients with CKD in the US. However, the real associations between blood Se and lipid profiles in this population should be verified in future prospective and randomized trials.


Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available at NHANES website, https://www.cdc.
References
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z (2019) Lipid accumulation and chronic kidney disease. Nutrients 11(4):722. https://doi.org/10.3390/nu11040722
Hager MR, Narla AD, Tannock LR (2017) Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 18(1):29–40. https://doi.org/10.1007/s11154-016-9402-z
Zubovic SV, Kristic S, Prevljak S, Pasic IS (2016) Chronic kidney disease and lipid disorders. Med Arch 70(3):191–192. https://doi.org/10.5455/medarh.2016.70.191-192
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6:25–54. https://doi.org/10.1039/c3mt00185g
Burk RF (2002) Selenium, an antioxidant nutrient. Nutr Clin Care 5:75–79. https://doi.org/10.1046/j.1523-5408.2002.00006.x
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Koller LD, Exon JH (1986) The two faces of selenium-deficiency and toxicity–are similar in animals and man. Can J Vet Res 50(3):297–306
Lubos E, Sinning CR, Schnabel RB, Wild PS, Zeller T, Rupprecht HJ et al (2010) Serum selenium and prognosis in cardiovascular disease: results from the AtheroGene study. Atherosclerosis 209(1):271–277. https://doi.org/10.1016/j.atherosclerosis.2009.09.008
Xiang S, Dai Z, Man C, Fan Y (2020) Circulating selenium and cardiovascular or all-cause mortality in the general population: a meta-analysis. Biol Trace Elem Res 195(1):55–62. https://doi.org/10.1007/s12011-019-01847-8
Vinceti M, Filippini T, Wise LA, Rothman KJ (2021) A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ Res 197:111210. https://doi.org/10.1016/j.envres.2021.111210
Yarmolinsky J, Bonilla C, Haycock PC, Langdon RJQ, Lotta LA, Langenberg C et al (2018) Circulating selenium and prostate cancer risk: a mendelian randomization analysis. J Natl Cancer Inst 110(9):1035–1038. https://doi.org/10.1093/jnci/djy081
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4(2B):593–599. https://doi.org/10.1079/phn2001143
Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E (2011) Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med 154(10):656–665. https://doi.org/10.7326/0003-4819-154-10-201105170-00005
Huang YQ, Shen G, Lo K, Huang JY, Liu L, Chen CL et al (2020) Association of circulating selenium concentration with dyslipidemia: Results from the NHANES. J Trace Elem Med Biol 58:126438. https://doi.org/10.1016/j.jtemb.2019.126438
Omrani H, Golmohamadi S, Pasdar Y, Jasemi K, Almasi A (2016) Effect of selenium supplementation on lipid profile in hemodialysis patients. J Renal Inj Prev 5(4):179–82. https://doi.org/10.15171/jrip.2016.38
Xie C, Zeng M, Shi Z, Li S, Jiang K, Zhao Y (2022) Association between selenium status and chronic kidney disease in middle-aged and older chinese based on CHNS data. Nutrients 14(13):2695. https://doi.org/10.3390/nu14132695
Wu CY, Wong CS, Chung CJ, Wu MY, Huang YL, Ao PL et al (2019) The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J Hazard Mater 375:224–232. https://doi.org/10.1016/j.jhazmat.2019.04.082
Centers for Disease Control and Prevention (CDC), NHANES—history [Internet]. https://www.cdc.gov/nchs/nhanes/history.htm. Accessed 10 Sep 2022
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group (2021) KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 100(4S):S1–S276. https://doi.org/10.1016/j.kint.2021.05.021
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez Á et al (2020) Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol 76(23):2712–2724. https://doi.org/10.1016/j.jacc.2020.10.008
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H et al (2016) 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 253:281–344. https://doi.org/10.1016/j.atherosclerosis.2016.08.018
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106(25):3143–3421
Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AF (2012) Endocrine society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:2969–2989. https://doi.org/10.1210/jc.2011-3213
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, ESC Scientific Document Group et al (2020) 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41(1):111–188. https://doi.org/10.1093/eurheartj/ehz455
Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis et al (2012) American association of clinical endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract 18 Suppl 1:1–78. https://doi.org/10.4158/ep.18.s1.1
American Diabetes Association Professional Practice Committee 2 Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 45((Suppl. S1)):S17–S38. https://doi.org/10.2337/dc22-S002
Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U (2012) Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380(9838):247–257. https://doi.org/10.1016/S0140-6736(12)60646-1
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30(4):377–399. https://doi.org/10.1002/sim.4067
Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P et al (2018) Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 14(12):727–749. https://doi.org/10.1038/s41581-018-0072-9
Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, SHARP Investigators et al (2011) The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377(9784):2181–92. https://doi.org/10.1016/S0140-6736(11)60739-3
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298(3):299–308. https://doi.org/10.1001/jama.298.3.299
Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A et al (2021) Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J 42(42):4324–4332. https://doi.org/10.1093/eurheartj/ehab432
Yan P, Xu Y, Miao Y, Bai X, Wu Y, Tang Q et al (2021) Association of remnant cholesterol with chronic kidney disease in middle-aged and elderly Chinese: a population-based study. Acta Diabetol 58(12):1615–1625. https://doi.org/10.1007/s00592-021-01765-z
Yu D, Wang Z, Zhang X, Qu B, Cai Y, Ma S et al (2021) Remnant cholesterol and cardiovascular mortality in patients with Type 2 diabetes and incident diabetic nephropathy. J Clin Endocrinol Metab 106(12):3546–3554. https://doi.org/10.1210/clinem/dgab533
Minich WB (2022) Selenium metabolism and biosynthesis of selenoproteins in the human body. Biochemistry (Mosc) 87(Suppl 1):S168–S102. https://doi.org/10.1134/S0006297922140139
Bunch DR, Cieslak W, Wang S (2017) Whole blood selenium determination by inductively coupled plasma mass spectrometry. Clin Biochem 50(12):710–713. https://doi.org/10.1016/j.clinbiochem.2017.01.013
Zachara BA, Salak A, Koterska D, Manitius J, Wasowicz W (2004) Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J Trace Elem Med Biol 17(4):291–299. https://doi.org/10.1016/S0946-672X(04)80031-2
Zachara BA (2015) Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem 68:131–151. https://doi.org/10.1016/bs.acc.2014.11.006
Fu S, Zhang L, Ma F, Xue S, Sun T, Xu Z (2022) Effects of selenium on chronic kidney disease: a mendelian randomization study. Nutrients 14(21):4458. https://doi.org/10.3390/nu14214458
Christensen K, Werner M, Malecki K (2015) Serum selenium and lipid levels: Associations observed in the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Environ Res 140:76–84. https://doi.org/10.1016/j.envres.2015.03.020
Ju W, Ji M, Li X, Li Z, Wu G, Fu X et al (2018) Relationship between higher serum selenium level and adverse blood lipid profile. Clin Nutr 37(5):1512–1517. https://doi.org/10.1016/j.clnu.2017.08.025
Stranges S, Laclaustra M, Ji C, Cappuccio FP, Navas-Acien A, Ordovas JM et al (2010) Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr 140(1):81–87. https://doi.org/10.3945/jn.109.111252
Cold F, Winther KH, Pastor-Barriuso R, Rayman MP, Guallar E, Nybo M et al (2015) Randomised controlled trial of the effect of long-term selenium supplementation on plasma cholesterol in an elderly Danish population. Br J Nutr 114(11):1807–1818. https://doi.org/10.1017/S0007114515003499
Wu Q, Sun X, Chen Q, Zhang X, Zhu Y (2021) Genetically predicted selenium is negatively associated with serum TC, LDL-C and positively associated with HbA1C levels. J Trace Elem Med Biol 67:126785. https://doi.org/10.1016/j.jtemb.2021.126785
Tabrizi R, Akbari M, Moosazadeh M, Lankarani KB, Heydari ST, Kolahdooz F et al (2017) The effects of selenium supplementation on glucose metabolism and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 49(11):826–830. https://doi.org/10.1055/s-0043-119544
Cobo G, Lindholm B, Stenvinkel P (2018) Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant 33(suppl_3):iii35–iii40. https://doi.org/10.1093/ndt/gfy175
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651
Duntas LH (2009) Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res 41(6):443–447. https://doi.org/10.1055/s-0029-1220724
Yu XH, Zheng XL, Tang CK (2015) Nuclear Factor-κB activation as a pathological mechanism of lipid metabolism and atherosclerosis. Adv Clin Chem 70:1–30. https://doi.org/10.1016/bs.acc.2015.03.004
Samuel VT, Petersen KF, Shulman GI (2010) Shulman, Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375(9733):2267–2277. https://doi.org/10.1016/S0140-6736(10)60408-4
Spoto B, Pisano A, Zoccali C (2016) Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 311(6):F1087–F1108. https://doi.org/10.1152/ajprenal.00340
Cardoso BR, Braat S, Graham RM (2021) Selenium status is associated with insulin resistance markers in adults: findings from the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES). Front Nutr 8:696024. https://doi.org/10.3389/fnut.2021.696024
Robberecht H, De Bruyne T, Davioud-Charvet E, Mackrill J, Hermans N (2019) Selenium status in elderly people: longevity and age-related diseases. Curr Pharm Des 25(15):1694–1706. https://doi.org/10.2174/1381612825666190701144709
Guo L, Yang W, Huang Q, Qiang J, Hart JR, Wang W et al (2018) Selenocysteine-specific mass spectrometry reveals tissue-distinct selenoproteomes and candidate selenoproteins. Cell Chem Biol 25(11):1380–1388. https://doi.org/10.1016/j.chembiol.2018.08.006
Suzuki KT, Kurasaki K, Okazaki N, Ogra Y (2005) Selenosugar and trimethylselenonium among urinary Se metabolites: dose and age-related changes. Toxicol Appl Pharmacol 206(1):1–8. https://doi.org/10.1016/j.taap.2004.10.018
Donadio JLS, Duarte GBS, Borel P, Cozzolino SMF, Rogero MM (2021) The influence of nutrigenetics on biomarkers of selenium nutritional status. Nutr Rev 79(11):1259–1273. https://doi.org/10.1093/nutrit/nuaa136
Acknowledgements
We thank the NHANES participants and staff for their contributions of the data and data collection.
Funding
This work was supported by the Guangzhou Municipal Health Commission under Grant 20221A011018.
Author information
Authors and Affiliations
Contributions
Ziyuan Li: Conceptualization, Methodology, Investigation, Data curation, Writing original draft; Jiahui Lai: Conceptualization, Software, Investigation, Writing – review & editing; Luona Wen: Investigation, Data curation; Qiongmei Chen:Investigation, Data curation; Rongshao Tan: Investigation, Writing–review & editing, Supervision; Xiaoshi Zhong: Investigation, Writing – review & editing, Supervision; Yun Liu: Methodology, Formal analysis, Project administration, Writing – review&editing, Supervision, Funding acquisition;Yan Liu: Conceptualization, Methodology, Formal analysis, Project administration, Writing – review & editing, Supervision.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Study protocols for NHANES were approved by the NCHS ethnics review board (Protocol #2011–17, https://www.cdc.gov/nchs/nhanes/irba98.htm). All the participants signed the informed consent before participating in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Lai, J., Wen, L. et al. Higher Levels of Blood Selenium are Associated with Higher Levels of Serum Lipid Profile in US Adults with CKD: Results from NHANES 2013–2018. Biol Trace Elem Res 201, 5501–5511 (2023). https://doi.org/10.1007/s12011-023-03608-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03608-0